Contractile performance of the Alaska blackfish (Dallia pectoralis) ventricle: Assessment of the effects of temperature, pacing frequency, the role of the sarcoplasmic reticulum in contraction and adrenergic stimulation
- PMID: 31493554
- PMCID: PMC6824994
- DOI: 10.1016/j.cbpa.2019.110564
Contractile performance of the Alaska blackfish (Dallia pectoralis) ventricle: Assessment of the effects of temperature, pacing frequency, the role of the sarcoplasmic reticulum in contraction and adrenergic stimulation
Abstract
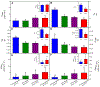
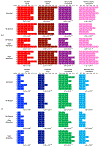
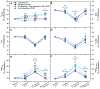
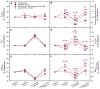
The air-breathing Alaska blackfish (Dallia pectoralis) experiences aquatic hypoxia, but restricted air-access in winter due to ice-cover. To lend insight into its overwintering strategy, we examined the effects of thermal acclimation (15 °C vs. 5 °C), acute temperature change (to 10 °C), increased pacing frequency, inhibition of sarcoplasmic reticulum (SR) Ca2+ release and uptake and adrenaline (1000 nmol l-1) on the contractile performance of isometrically-contracting, electrically-paced ventricular strips. At routine pacing frequencies, maximal developed force (Fmax) was equivalent at 5 °C (2.1 ± 0.2 mN mm-2) and 15 °C (2.2 ± 0.3 mN mm-2), whereas contraction durations were 2.2- to 2.4-times longer and contraction rates 2.4- to 3.5-times slower at 5 °C. Maximum contraction frequency was reduced by decreased temperature, being 0.91 ± 0.04 Hz at 15 °C, 0.35 ± 0.02 Hz at 5 °C and equivalent between acclimation groups at 10 °C (~0.8 Hz). 15 °C and 5 °C strips were insensitive to SR inhibition at routine stimulation frequencies, but SR function supported high contraction rates at 10 °C and 15 °C. Adrenaline shortened T0.5R and increased relaxation rate by 18-40% at 15 °C, whereas at 5 °C, adrenaline augmented Fmax by 15-25%, in addition to increasing contraction kinetics by 22-82% and decreasing contraction duration by 20%. Overall, the results reveal that ventricular contractility is suppressed in cold-acclimated Alaska blackfish largely by acute and perhaps direct effects of decreased temperature, which effectively preconditions the tissue for low energy supply during winter hypoxia. Additionally, the level of cardiac performance associated with maintained activity in winter is supported by enhanced inotropic responsiveness to adrenaline at 5 °C.
Keywords: Adrenaline; Cardiac; Force-frequency; Heart; Ryanodine; Sarcoplasmic reticulum; Temperature; Thapsigargin.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest
The authors declare no competing interests.
Figures




References
-
- Abramochkin DV, Vornanen M, 2015. Seasonal acclimatization of the cardiac potassium currents (IK1 and IKr) in an arctic marine teleost, the navaga cod (Eleginus navaga). J. Comp. Physiol. B 185, 883–890. - PubMed
-
- Aho E, Vornanen M, 1999. Contractile properties of atrial and ventricular myocardium of the heart of rainbow trout Oncorhynchus mykiss: Effects of thermal acclimation. J. Exp. Biol 202, 2663–2677. - PubMed
-
- Aho E, Vornanen M, 2001. Cold acclimation increases basal heart rate but decreases its thermal tolerance in rainbow trout (Oncorhynchus mykiss). J. Comp. Phyisol. B 171, 173–179. - PubMed
-
- Birkedal R, Shiels HA, 2007. High [Na+]i in cardiomyocytes from rainbow trout. Am. J. Phys. Regul. Integr. Comp. Phys 293, R861–R866. - PubMed
-
- Campbell MA, Lopéz JA, 2014. Mitochondrial phylogeography of a Beringian relict: the endemic freshwater genus of blackfish Dallia (Esociformes). J. Fish Biol. 84, 523–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

