Bioactive proteins delivery through core-shell nanofibers for meniscal tissue regeneration
- PMID: 31493556
- PMCID: PMC6911641
- DOI: 10.1016/j.nano.2019.102090
Bioactive proteins delivery through core-shell nanofibers for meniscal tissue regeneration
Abstract
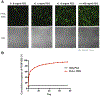
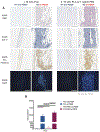
Mimicking the ultrastructural morphology of the meniscus with nanofiber scaffolds, coupled with controlled growth-factor delivery to the appropriate cells, can help engineer tissue with the potential to grow, mature, and regenerate after in vivo implantation. We electrospun nanofibers encapsulating platelet-derived growth factor (PDGF-BB), which is a potent mitogen and chemoattractant in a core of serum albumin contained within a shell of polylactic acid. We controlled the local PDGF-BB release by adding water-soluble polyethylene glycol to the polylactic acid shell to serve as a porogen. The novel core-shell nanofibers generated 3D scaffolds with an interconnected macroporous structure, with appropriate mechanical properties and with high cell compatibility. Incorporating PDGF-BB increased cell viability, proliferation, and infiltration, and upregulated key genes involved in meniscal extracellular matrix synthesis in human meniscal and synovial cells. Our results support proof of concept that these core-shell nanofibers can create a cell-favorable nanoenvironment and can serve as a system for sustained release of bioactive factors.
Keywords: Co-axial electrospinning; Core-shell structure; Meniscus; Nanofibers; PDGF-BB; Tissue engineering.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures





References
-
- Hickey DS, Hukins DW. Relation between the structure of the annulus fibrosus and the function and failure of the intervertebral disc. Spine (Phila Pa 1976) 1980;5:106–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

