ABT-737 Triggers Caspase-Dependent Inhibition of Platelet Procoagulant Extracellular Vesicle Release during Apoptosis and Secondary Necrosis In Vitro
- PMID: 31493778
- PMCID: PMC6768798
- DOI: 10.1055/s-0039-1693694
ABT-737 Triggers Caspase-Dependent Inhibition of Platelet Procoagulant Extracellular Vesicle Release during Apoptosis and Secondary Necrosis In Vitro
Erratum in
-
Erratum.Thromb Haemost. 2019 Oct;119(10):e1. doi: 10.1055/s-0040-1702204. Epub 2020 May 18. Thromb Haemost. 2019. PMID: 32422665 No abstract available.
Abstract
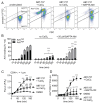
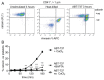
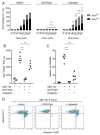
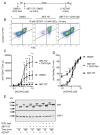
Platelet lifespan is limited by activation of intrinsic apoptosis. Apoptotic platelets are rapidly cleared from the circulation in vivo. ABT-737 triggers platelet apoptosis and is a useful tool for studying this process. However, in vitro experiments lack clearance mechanisms for apoptotic platelets. To determine whether apoptotic platelets progress to secondary necrosis, apoptosis was triggered in human platelets with ABT-737, a BH3 mimetic. Platelet annexin V (AnV) binding, release of AnV+ extracellular vesicles (EVs), and loss of plasma membrane integrity were monitored by flow cytometry. ABT-737 triggered AnV binding, indicating phosphatidylserine exposure, release of AnV+ EVs, and a slow loss of plasma membrane integrity. The latter suggests that apoptotic platelets progress to secondary necrosis in vitro. These responses were dependent on caspase activation and Ca2+ entry. Surprisingly, although intracellular Ca2+ concentration increased, AnV+ EV release was not dependent on the Ca2+-dependent protease, calpain. On the contrary, ABT-737 downregulated the ability of the Ca2+ ionophore, A23187, to trigger calpain-dependent release of AnV+ EVs. This was dependent on caspase activity as, when caspases were inhibited, ABT-737 increased the ability of A23187 to trigger AnV+ EV release. These data suggest that apoptotic platelets progress to secondary necrosis unless they are cleared. This may affect the interpretation of ABT-737-triggered signaling in platelets in vitro. Ca2+-dependent AnV+ EV release is downregulated during apoptosis in a caspase-dependent manner, which may limit the potential consequences of secondary necrotic platelets.
Georg Thieme Verlag KG Stuttgart · New York.
Conflict of interest statement
None declared.
Figures

Similar articles
-
Activation of caspases-9, -3 and -8 in human platelets triggered by BH3-only mimetic ABT-737 and calcium ionophore A23187: caspase-8 is activated via bypass of the death receptors.Br J Haematol. 2012 Dec;159(5):565-71. doi: 10.1111/bjh.12066. Epub 2012 Oct 1. Br J Haematol. 2012. PMID: 23025479
-
BH3-mimetic ABT-737 induces strong mitochondrial membrane depolarization in platelets but only weakly stimulates apoptotic morphological changes, platelet shrinkage and microparticle formation.Thromb Res. 2014 Jan;133(1):73-9. doi: 10.1016/j.thromres.2013.10.041. Epub 2013 Nov 6. Thromb Res. 2014. PMID: 24268298
-
Inner Mitochondrial Membrane Disruption Links Apoptotic and Agonist-Initiated Phosphatidylserine Externalization in Platelets.Arterioscler Thromb Vasc Biol. 2017 Aug;37(8):1503-1512. doi: 10.1161/ATVBAHA.117.309473. Epub 2017 Jun 29. Arterioscler Thromb Vasc Biol. 2017. PMID: 28663253 Free PMC article.
-
Concurrent and separate inside-out transition of platelet apoptosis and activation markers to the platelet surface.Br J Haematol. 2013 Nov;163(3):377-84. doi: 10.1111/bjh.12529. Epub 2013 Aug 23. Br J Haematol. 2013. PMID: 24033315
-
P2Y12 protects platelets from apoptosis via PI3k-dependent Bak/Bax inactivation.J Thromb Haemost. 2013 Jan;11(1):149-60. doi: 10.1111/jth.12063. J Thromb Haemost. 2013. PMID: 23140172
Cited by
-
Carbamazepine Induces Platelet Apoptosis and Thrombocytopenia Through Protein Kinase A.Front Pharmacol. 2021 Sep 23;12:749930. doi: 10.3389/fphar.2021.749930. eCollection 2021. Front Pharmacol. 2021. PMID: 34658890 Free PMC article.
-
Procoagulant Phosphatidylserine-Exposing Platelets in vitro and in vivo.Front Cardiovasc Med. 2020 Mar 3;7:15. doi: 10.3389/fcvm.2020.00015. eCollection 2020. Front Cardiovasc Med. 2020. PMID: 32195268 Free PMC article. Review.
-
Cytosolic and mitochondrial Ca2+ signaling in procoagulant platelets.Platelets. 2021 Oct 3;32(7):855-862. doi: 10.1080/09537104.2021.1881951. Epub 2021 Feb 18. Platelets. 2021. PMID: 33600275 Free PMC article.
-
Identification of platelet subpopulations in cryopreserved platelet components using multi-colour imaging flow cytometry.Sci Rep. 2023 Jan 21;13(1):1221. doi: 10.1038/s41598-023-28352-2. Sci Rep. 2023. PMID: 36681723 Free PMC article.
-
2-Aminoethoxydiphenylborate (2-APB) inhibits release of phosphatidylserine-exposing extracellular vesicles from platelets.Cell Death Discov. 2020 Mar 2;6:10. doi: 10.1038/s41420-020-0244-9. eCollection 2020. Cell Death Discov. 2020. PMID: 32140260 Free PMC article.
References
-
- Lebois M, Josefsson EC. Regulation of platelet lifespan by apoptosis. Platelets. 2016;27(06):497–504. - PubMed
-
- Kile BT. The role of apoptosis in megakaryocytes and platelets. Br J Haematol. 2014;165(02):217–226. - PubMed
-
- Mason KD, Carpinelli MR, Fletcher JI, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128(06):1173–1186. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

