Asymmetric Bilayers by Hemifusion: Method and Leaflet Behaviors
- PMID: 31493862
- PMCID: PMC6818168
- DOI: 10.1016/j.bpj.2019.07.054
Asymmetric Bilayers by Hemifusion: Method and Leaflet Behaviors
Abstract
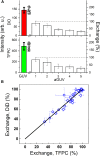
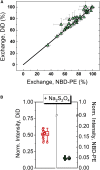
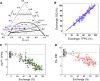
We describe a new method to prepare asymmetric giant unilamellar vesicles (aGUVs) via hemifusion. Hemifusion of giant unilamellar vesicles and a supported lipid bilayer, triggered by calcium, promotes the lipid exchange of the fused outer leaflets mediated by lipid diffusion. We used different fluorescent dyes to monitor the inner and the outer leaflets of the unsupported aGUVs. We confirmed that almost all newly exchanged lipids in the aGUVs are found in the outer leaflet of these asymmetric vesicles. In addition, we test the stability of the aGUVs formed by hemifusion in preserving their contents during the procedure. For aGUVs prepared from the hemifusion of giant unilamellar vesicles composed of 1,2-distearoyl-sn-glycero-3-phosphocholine/1,2-dioleoyl-sn-glycero-3-phosphocholine/cholesterol = 0.39/0.39/0.22 and a supported lipid bilayer of 1,2-dioleoyl-sn-glycero-3-phosphocholine/cholesterol = 0.8/0.2, we observed the exchanged lipids to alter the bilayer properties. To access the physical and chemical properties of the asymmetric bilayer, we monitored the dye partition coefficients of individual leaflets and the generalized polarization of the fluorescence probe 6-dodecanoyl-2-[ N-methyl-N-(carboxymethyl)amino] naphthalene, a sensor for the lipid packing/order of its surroundings. For a high percentage of lipid exchange (>70%), the dye partition indicates induced-disordered and induced-ordered domains. The induced domains have distinct lipid packing/order compared to the symmetric liquid-disordered and liquid-ordered domains.
Copyright © 2019 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures





Comment in
-
Kiss and Run Asymmetric Vesicles to Investigate Coupling.Biophys J. 2019 Sep 17;117(6):1009-1011. doi: 10.1016/j.bpj.2019.07.053. Epub 2019 Aug 21. Biophys J. 2019. PMID: 31477242 Free PMC article. No abstract available.
Similar articles
-
The use of hemifusion to create asymmetric giant unilamellar vesicles: Insights on induced order domains.Methods Enzymol. 2024;700:127-159. doi: 10.1016/bs.mie.2024.03.025. Epub 2024 Apr 6. Methods Enzymol. 2024. PMID: 38971598
-
Investigation of the domain line tension in asymmetric vesicles prepared via hemifusion.Biochim Biophys Acta Biomembr. 2021 Jun 1;1863(6):183586. doi: 10.1016/j.bbamem.2021.183586. Epub 2021 Feb 26. Biochim Biophys Acta Biomembr. 2021. PMID: 33647248 Free PMC article.
-
Experimentally determined leaflet-leaflet phase diagram of an asymmetric lipid bilayer.Proc Natl Acad Sci U S A. 2023 Nov 14;120(46):e2308723120. doi: 10.1073/pnas.2308723120. Epub 2023 Nov 8. Proc Natl Acad Sci U S A. 2023. PMID: 37939082 Free PMC article.
-
Indirect evidence of submicroscopic pores in giant unilamellar [correction of unilamelar] vesicles.Biochim Biophys Acta. 2005 Aug 5;1724(3):281-7. doi: 10.1016/j.bbagen.2005.04.028. Biochim Biophys Acta. 2005. PMID: 15978732 Review.
-
Lipid packing determines protein-membrane interactions: challenges for apolipoprotein A-I and high density lipoproteins.Biochim Biophys Acta. 2010 Jul;1798(7):1399-408. doi: 10.1016/j.bbamem.2010.03.019. Epub 2010 Mar 27. Biochim Biophys Acta. 2010. PMID: 20347719 Free PMC article. Review.
Cited by
-
Nano-scale domains in the plasma membrane are like macroscopic domains in asymmetric bilayers.Biophys J. 2023 Mar 21;122(6):925-930. doi: 10.1016/j.bpj.2022.11.020. Epub 2022 Nov 14. Biophys J. 2023. PMID: 36380589 Free PMC article. Review.
-
Biomimetic asymmetric bacterial membranes incorporating lipopolysaccharides.Biophys J. 2023 Jun 6;122(11):2147-2161. doi: 10.1016/j.bpj.2022.12.017. Epub 2022 Dec 15. Biophys J. 2023. PMID: 36523159 Free PMC article.
-
Lipid Scrambling Induced by Membrane-Active Substances.Biophys J. 2020 Aug 18;119(4):767-779. doi: 10.1016/j.bpj.2020.07.004. Epub 2020 Jul 14. Biophys J. 2020. PMID: 32738218 Free PMC article.
-
Kiss and Run Asymmetric Vesicles to Investigate Coupling.Biophys J. 2019 Sep 17;117(6):1009-1011. doi: 10.1016/j.bpj.2019.07.053. Epub 2019 Aug 21. Biophys J. 2019. PMID: 31477242 Free PMC article. No abstract available.
-
Developing initial conditions for simulations of asymmetric membranes: a practical recommendation.Biophys J. 2021 Nov 16;120(22):5041-5059. doi: 10.1016/j.bpj.2021.10.009. Epub 2021 Oct 13. Biophys J. 2021. PMID: 34653389 Free PMC article.
References
-
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

