Mitochondria and Critical Illness
- PMID: 31494084
- PMCID: PMC7005375
- DOI: 10.1016/j.chest.2019.08.2182
Mitochondria and Critical Illness
Abstract
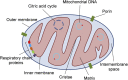
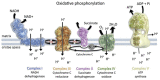
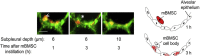
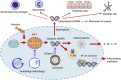
Classically, mitochondria have largely been believed to influence the development of illness by modulating cell metabolism and determining the rate of production of high-energy phosphate compounds (eg, adenosine triphosphate). It is now recognized that this view is simplistic and that mitochondria play key roles in many other processes, including cell signaling, regulating gene expression, modulating cellular calcium levels, and influencing the activation of cell death pathways (eg, caspase activation). Moreover, these multiple mitochondrial functional characteristics are now known to influence the evolution of cellular and organ function in many disease states, including sepsis, ICU-acquired skeletal muscle dysfunction, acute lung injury, acute renal failure, and critical illness-related immune function dysregulation. In addition, diseased mitochondria generate toxic compounds, most notably released mitochondrial DNA, which can act as danger-associated molecular patterns to induce systemic toxicity and damage multiple organs throughout the body. This article reviews these evolving concepts relating mitochondrial function and acute illness. The discussion is organized into four sections: (1) basics of mitochondrial physiology; (2) cellular mechanisms of mitochondrial pathophysiology; (3) critical care disease processes whose initiation and evolution are shaped by mitochondrial pathophysiology; and (4) emerging treatments for mitochondrial dysfunction in critical illness.
Keywords: critical illness; lung injury; mitochondria; muscle dysfunction; sepsis.
Copyright © 2019 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Rongvaux A. Innate immunity and tolerance toward mitochondria. Mitochondrion. 2018;41:14–20. - PubMed
-
- Abate M, Festa A, Falco M, et al. Mitochondria as playmakers of apoptosis, autophagy and senescence [published online ahead of print June 26, 2019]. Semin Cell Dev Biol. doi.org/10.1016/j.semcdb.2019.05.022. - PubMed
-
- Davies K.M., Daum B. Role of cryo-ET in membrane bioenergetics research. Biochem Soc Trans. 2013;41:1227–1234. - PubMed

