Effectiveness of influenza vaccines in adults with chronic liver disease: a systematic review and meta-analysis
- PMID: 31494620
- PMCID: PMC6731888
- DOI: 10.1136/bmjopen-2019-031070
Effectiveness of influenza vaccines in adults with chronic liver disease: a systematic review and meta-analysis
Abstract
Objectives: Patients with liver disease frequently require hospitalisation with infection often the trigger. Influenza vaccination is an effective infection prevention strategy in healthy and elderly but is often perceived less beneficial in patients with liver disease. We investigated whether influenza vaccination triggered serological response and prevented hospitalisation and death in liver disease.
Design: Systematic review and meta-analysis.
Data sources: MEDLINE, EMBASE, PubMed and CENTRAL up to January 2019.
Eligibility criteria: Randomised or observational studies of the effects of influenza vaccine in adults with liver disease.
Data extraction and synthesis: Two reviewers screened studies, extracted data and assessed risk of bias and quality of evidence. Primary outcomes were all-cause hospitalisation and mortality. Secondary outcomes were cause-specific hospitalisation and mortality, and serological vaccine response. Random-effects meta-analysis was used to estimate pooled effects of vaccination.
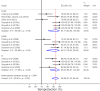
Results: We found 10 041 unique records, 286 were eligible for full-text review and 12 were included. Most patients had viral liver disease. All studies were of very low quality. Liver patients both with and without cirrhosis mounted an antibody response to influenza vaccination, and vaccination was associated with a reduction in risk of hospital admission from 205/1000 to 149/1000 (risk difference -0.06, 95% CI -0.07 to 0.04) in patients with viral liver disease. Vaccinated patients were 27% less likely to be admitted to hospital compared with unvaccinated patients (risk ratio 0.73, 95% CI 0.66 to 0.80). No effect against all-cause or cause-specific mortality or cause-specific hospitalisation was found.
Conclusions: The low quantity and quality of the evidence means that the protective vaccine effect may be uncertain. Considering the high risk of serious health outcomes from influenza infection in patients with liver disease and the safety and low cost of vaccination, overall, the potential benefits of seasonal vaccination both to patients and the healthcare systems are likely to outweigh the costs and risks associated with vaccination.
Prospero registration number: CRD42017067277.
Keywords: cirrhosis; hospitalisation; influenza vaccine; liver disease; seroprotection; vaccine effectiveness.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





References
-
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet 2017;390:1211–59. 10.1016/S0140-6736(17)32154-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
