Delay in booster schedule as a control parameter in vaccination dynamics
- PMID: 31494722
- PMCID: PMC6858909
- DOI: 10.1007/s00285-019-01424-6
Delay in booster schedule as a control parameter in vaccination dynamics
Abstract
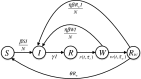
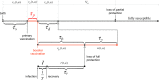
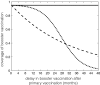
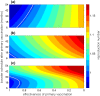
The use of multiple vaccine doses has proven to be essential in providing high levels of protection against a number of vaccine-preventable diseases at the individual level. However, the effectiveness of vaccination at the population level depends on several key factors, including the dose-dependent protection efficacy of vaccine, coverage of primary and booster doses, and in particular, the timing of a booster dose. For vaccines that provide transient protection, the optimal scheduling of a booster dose remains an important component of immunization programs and could significantly affect the long-term disease dynamics. In this study, we developed a vaccination model as a system of delay differential equations to investigate the effect of booster schedule using a control parameter represented by a fixed time-delay. By exploring the stability analysis of the model based on its reproduction number, we show the disease persistence in scenarios where the booster dose is sub-optimally scheduled. The findings indicate that, depending on the protection efficacy of primary vaccine series and the coverage of booster vaccination, the time-delay in a booster schedule can be a determining factor in disease persistence or elimination. We present model results with simulations for a vaccine-preventable bacterial disease, Heamophilus influenzae serotype b, using parameter estimates from the previous literature. Our study highlights the importance of timelines for multiple-dose vaccination in order to enhance the population-wide benefits of herd immunity.
Keywords: Booster schedule; Delay equations; Persistence; Reproduction number; Vaccination.
Figures
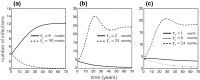
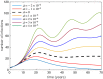
References
-
- Briere EC, Rubin L, Moro PL, Cohn A, Clark T, Messonnier N, et al. Prevention and control of haemophilus influenzae type b disease: recommendations of the advisory committee on immunization practices (acip) MMWR Recomm Rep. 2014;63(RR–01):1–14. - PubMed
-
- Centers for Disease Control and Prevention et al (2009) Invasive haemophilus influenzae type b disease in five young children–Minnesota. Ann Emerg Med 54(1):83–85 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

