Reactive Gliosis Contributes to Nrf2-Dependent Neuroprotection by Pretreatment with Dimethyl Fumarate or Korean Red Ginseng Against Hypoxic-Ischemia: Focus on Hippocampal Injury
- PMID: 31494826
- PMCID: PMC6980429
- DOI: 10.1007/s12035-019-01760-0
Reactive Gliosis Contributes to Nrf2-Dependent Neuroprotection by Pretreatment with Dimethyl Fumarate or Korean Red Ginseng Against Hypoxic-Ischemia: Focus on Hippocampal Injury
Abstract
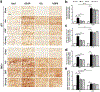
Recently, dimethyl fumarate (DMF) and Korean red ginseng (ginseng), based on their purported antioxidative and anti-inflammatory properties, have exhibited protective potential in various neurological conditions. Their effects on cerebral ischemia and underlying mechanisms remain inconclusive; however, increasing evidence indicates the involvement of the transcriptional factor Nrf2. This study evaluated the preventive effects of DMF and ginseng on hippocampal neuronal damage following hypoxia-ischemia (HI) and assessed the contributions of reactive gliosis and the Nrf2 pathway. Adult wild type (WT) and Nrf2-/- mice were pretreated with DMF or ginseng for 7 days prior to HI. At 24 h after HI, DMF or ginseng significantly reduced infarct volume (52.5 ± 12.3% and 47.8 ± 10.7%), brain edema (61.5 ± 17.4% and 39.3 ± 12.8%), and hippocampal CA1 neuronal degeneration, and induced expressions of Nrf2 target proteins in WT, but not Nrf2-/-, mice. Such hippocampal neuroprotective benefits were also observed at 6 h and 7 days after HI. The dynamic attenuation of reactive gliosis in microglia and astrocytes correlated well with this sustained neuroprotection in an Nrf2-dependent manner. In both early and late stages of HI, astrocytic dysfunctions in extracellular glutamate clearance and water transport, as indicated by glutamine synthetase and aquaporin 4, were also attenuated after HI in WT, but not Nrf2-/-, mice treated with DMF or ginseng. Together, DMF and ginseng confer robust and prolonged Nrf2-dependent neuroprotection against ischemic hippocampal damage. The salutary Nrf2-dependent attenuation of reactive gliosis may contribute to this neuroprotection, offering new insight into the cellular basis of an Nrf2-targeting strategy for stroke prevention or treatment.
Keywords: Astrocyte; Astrogliosis; Microglia; Oxidative stress; Stroke; Transcriptional factor.
Conflict of interest statement
Author Disclosure Statement
The authors declare no conflicts of interests.
Figures

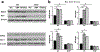


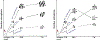
Similar articles
-
Pretreatment with Korean red ginseng or dimethyl fumarate attenuates reactive gliosis and confers sustained neuroprotection against cerebral hypoxic-ischemic damage by an Nrf2-dependent mechanism.Free Radic Biol Med. 2019 Feb 1;131:98-114. doi: 10.1016/j.freeradbiomed.2018.11.017. Epub 2018 Nov 17. Free Radic Biol Med. 2019. PMID: 30458277 Free PMC article.
-
Nrf2 Plays an Essential Role in Long-Term Brain Damage and Neuroprotection of Korean Red Ginseng in a Permanent Cerebral Ischemia Model.Antioxidants (Basel). 2019 Aug 3;8(8):273. doi: 10.3390/antiox8080273. Antioxidants (Basel). 2019. PMID: 31382635 Free PMC article.
-
Korean Red Ginseng Pretreatment Protects Against Long-Term Sensorimotor Deficits After Ischemic Stroke Likely Through Nrf2.Front Cell Neurosci. 2018 Mar 23;12:74. doi: 10.3389/fncel.2018.00074. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29628876 Free PMC article.
-
Argon neuroprotection in ischemic stroke and its underlying mechanism.Brain Res Bull. 2024 Jun 15;212:110964. doi: 10.1016/j.brainresbull.2024.110964. Epub 2024 Apr 25. Brain Res Bull. 2024. PMID: 38670471 Review.
-
The cell-specific roles of Nrf2 in acute and chronic phases of ischemic stroke.CNS Neurosci Ther. 2024 Mar;30(3):e14462. doi: 10.1111/cns.14462. Epub 2023 Sep 16. CNS Neurosci Ther. 2024. PMID: 37715557 Free PMC article. Review.
Cited by
-
Dimethyl Fumarate Attenuates Lymphocyte Infiltration and Reduces Infarct Size in Experimental Stroke.Int J Mol Sci. 2023 Oct 24;24(21):15540. doi: 10.3390/ijms242115540. Int J Mol Sci. 2023. PMID: 37958527 Free PMC article.
-
The Antioxidant Transcription Factor Nrf2 in Cardiac Ischemia-Reperfusion Injury.Int J Mol Sci. 2021 Nov 3;22(21):11939. doi: 10.3390/ijms222111939. Int J Mol Sci. 2021. PMID: 34769371 Free PMC article. Review.
-
Trends in Gliosis in Obesity, and the Role of Antioxidants as a Therapeutic Alternative.Antioxidants (Basel). 2022 Oct 1;11(10):1972. doi: 10.3390/antiox11101972. Antioxidants (Basel). 2022. PMID: 36290695 Free PMC article. Review.
-
Activation of Nrf2 by Natural Bioactive Compounds: A Promising Approach for Stroke?Int J Mol Sci. 2020 Jul 10;21(14):4875. doi: 10.3390/ijms21144875. Int J Mol Sci. 2020. PMID: 32664226 Free PMC article. Review.
-
The Nrf2 Pathway in Ischemic Stroke: A Review.Molecules. 2021 Aug 18;26(16):5001. doi: 10.3390/molecules26165001. Molecules. 2021. PMID: 34443584 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

