Capping Protein Insulates Arp2/3-Assembled Actin Patches from Formins
- PMID: 31495586
- PMCID: PMC6864609
- DOI: 10.1016/j.cub.2019.07.088
Capping Protein Insulates Arp2/3-Assembled Actin Patches from Formins
Abstract
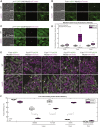
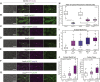
How actin structures of distinct identities and functions coexist within the same environment is a critical self-organization question. Fission yeast cells have a simple actin cytoskeleton made of four structures: Arp2/3 assembles actin patches around endocytic pits, and the formins For3, Cdc12, and Fus1 assemble actin cables, the cytokinetic ring during division, and the fusion focus during sexual reproduction, respectively. The focus concentrates the delivery of hydrolases by myosin V to digest the cell wall for cell fusion. We discovered that cells lacking capping protein (CP), a heterodimer that blocks barbed-end dynamics and associates with actin patches, exhibit a delay in fusion. Consistent with CP-formin competition for barbed-end binding, Fus1, F-actin, and the linear filament marker tropomyosin hyper-accumulate at the fusion focus in cells lacking CP. CP deletion also rescues the fusion defect of a mutation in the Fus1 knob region. However, myosin V and exocytic cargoes are reduced at the fusion focus and diverted to ectopic foci, which underlies the fusion defect. Remarkably, the ectopic foci coincide with Arp2/3-assembled actin patches, which now contain low levels of Fus1. We further show that CP localization to actin patches is required to prevent the formation of ectopic foci and promote efficient cell fusion. During mitotic growth, actin patches lacking CP similarly display a dual identity, as they accumulate the formins For3 and Cdc12, normally absent from patches, and are co-decorated by the linear filament-binding protein tropomyosin and the patch marker fimbrin. Thus, CP serves to protect Arp2/3-nucleated structures from formin activity.
Keywords: actin; actin homeostasis; actin patch; capping protein; cell-cell fusion; fission yeast Schizosaccharomyces pombe; formin.
Copyright © 2019 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

