Interactions among glomerulus infiltrating macrophages and intrinsic cells via cytokines in chronic lupus glomerulonephritis
- PMID: 31495649
- PMCID: PMC6930355
- DOI: 10.1016/j.jaut.2019.102331
Interactions among glomerulus infiltrating macrophages and intrinsic cells via cytokines in chronic lupus glomerulonephritis
Abstract
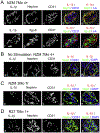
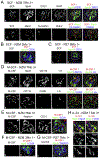
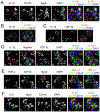
Inflammation plays a key role in the pathogenesis of lupus nephritis (LN) and inflammatory cytokines within the glomeruli are critical in this process. However, little information is available for the identities of the cell types that are primarily responsible for the production and function of the various cytokines. We have devised a novel method to visualize cytokine signals in the kidney by confocal microscopy and found that cytokine production within the glomerulus is cell type-specific and under translational control. In the lupus-prone NZM2328 mice with chronic glomerulonephritis, IL-6, IL-1β, and TNF-α in the glomerulus were produced predominantly by mesangial cells, podocytes, and glomerulus-infiltrating blood-derived macrophages, respectively. Microarray and RNASeq analyses showed that these cells expressed the receptors for these cytokines. Together the 3 cell types form a cytokine circuit in amplifying cytokine responses in LN. The intrinsic cells and infiltrating macrophages also produced other cytokines including M-CSF, SCF, and IL-34 that constituted within the enclosed glomerular space the soluble effector milieu which may mediate cellular damage and proliferation, and cytokine transcriptional and translation regulation. IL-10 and IL-1β were translationally regulated in the glomeruli in the intact kidney in a cell type-specific manner. The production of these 2 cytokines by infiltrating macrophages was undetectable in a visualization system for in situ protein accumulation despite high mRNA expression levels. However, these macrophages in isolated glomeruli which are released from Bowman's capsules produced large amounts of IL-10 and IL-1β. These data reveal the complexity of cytokine regulation, production, and function in the glomerulus and provide a model in which cytokine blocking may be beneficial in LN treatment.
Keywords: Cytokines; Lupus nephritis; Macrophages; Mesangial cells; Podocytes; Translational regulation.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures
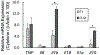



References
-
- Fu SM, Sung S.-s. J, Wang H, Gaskin F, Pathogenesis of Lupus Nephritis, in: Wallace D, Hahn B, (Eds.), Dubois’ Lupus Erythematosus and Related Syndromes, 9th Edition, Elsevier, New York, N.Y., 2019, pp. 269–293.
-
- Naito Y, Uchiyama K, Mizushima K, Kuroda M, Akagiri S, Takagi T et al., Microarray profiling of gene expression patterns in glomerular cells of astaxanthin-treated diabetic mice: a nutrigenomic approach, Int. J. Mol. Med 18 (2006) 685–695 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

