Preparation of a Series of Supported Nonsymmetrical PNP-Pincer Ligands and the Application in Ester Hydrogenation
- PMID: 31495988
- PMCID: PMC6916561
- DOI: 10.1002/chem.201903379
Preparation of a Series of Supported Nonsymmetrical PNP-Pincer Ligands and the Application in Ester Hydrogenation
Abstract
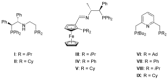
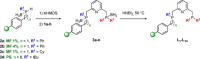
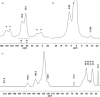
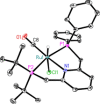
In contrast to their symmetrical analogues, nonsymmetrical PNP-type ligand motifs have been less investigated despite the modular pincer structure. However, the introduction of mixed phosphorus donor moieties provides access to a larger variety of PNP ligands. Herein, a facile solid-phase synthesis approach towards a diverse PNP-pincer ligand library of 14 members is reported. Contrary to often challenging workup procedures in solution-phase, only simple workup steps are required. The corresponding supported ruthenium-PNP catalysts are screened in ester hydrogenation. Usually, industrially applied heterogeneous catalysts require harsh conditions in this reaction (250-350 °C at 100-200 bar) often leading to reduced selectivities. Heterogenized reusable Ru-PNP catalysts are capable of reducing esters and lactones selectively under mild conditions.
Keywords: catalyst immobilization; heterogeneous catalysis; hydrogenation; pincer ligands; solid-phase synthesis.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

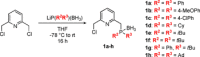







Similar articles
-
Unmasking the Ligand Effect in Manganese-Catalyzed Hydrogenation: Mechanistic Insight and Catalytic Application.J Am Chem Soc. 2019 Oct 30;141(43):17337-17349. doi: 10.1021/jacs.9b09038. Epub 2019 Oct 21. J Am Chem Soc. 2019. PMID: 31633346
-
Hydrogenation and dehydrogenation iron pincer catalysts capable of metal-ligand cooperation by aromatization/dearomatization.Acc Chem Res. 2015 Jul 21;48(7):1979-94. doi: 10.1021/acs.accounts.5b00027. Epub 2015 Jun 16. Acc Chem Res. 2015. PMID: 26079678
-
Modularly designed transition metal PNP and PCP pincer complexes based on aminophosphines: synthesis and catalytic applications.Acc Chem Res. 2008 Feb;41(2):201-13. doi: 10.1021/ar700129q. Epub 2008 Jan 23. Acc Chem Res. 2008. PMID: 18211031
-
Recent advances in osmium-catalyzed hydrogenation and dehydrogenation reactions.Acc Chem Res. 2015 Feb 17;48(2):363-79. doi: 10.1021/ar5003818. Epub 2015 Feb 4. Acc Chem Res. 2015. PMID: 25650714 Review.
-
Recent Advances in Chemistry of Unsymmetrical Phosphorus-Based Pincer Nickel Complexes: From Design to Catalytic Applications.Molecules. 2021 Jul 2;26(13):4063. doi: 10.3390/molecules26134063. Molecules. 2021. PMID: 34279402 Free PMC article. Review.
References
-
- None
-
- Moulton C. J., Shaw B. L., J. Chem. Soc. Dalton Trans. 1976, 1020;
-
- van Koten G., Timmer K., Noltes J. G., Spek A. L., J. Chem. Soc. Chem. Commun. 1978, 250;
-
- van der Vlugt J. I., Reek J. N., Angew. Chem. Int. Ed. 2009, 48, 8832; - PubMed
- Angew. Chem. 2009, 121, 8990;
-
- Gunanathan C., Milstein D., Chem. Rev. 2014, 114, 12024; - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

