Schistosoma japonicum peptide SJMHE1 suppresses airway inflammation of allergic asthma in mice
- PMID: 31496071
- PMCID: PMC6815837
- DOI: 10.1111/jcmm.14661
Schistosoma japonicum peptide SJMHE1 suppresses airway inflammation of allergic asthma in mice
Abstract
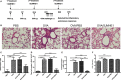
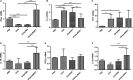
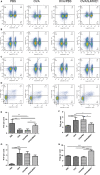
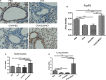
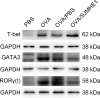
Helminths and their products can shape immune responses by modulating immune cells, which are dysfunctional in inflammatory diseases such as asthma. We previously identified SJMHE1, a small molecule peptide from the HSP60 protein of Schistosoma japonicum. SJMHE1 can inhibit delayed-type hypersensitivity and collagen-induced arthritis in mice. In the present study, we evaluated this peptide's potential intervention effect and mechanism on ovalbumin-induced asthma in mice. SJMHE1 treatment suppressed airway inflammation in allergic mice, decreased the infiltrating inflammatory cells in the lungs and bronchoalveolar lavage fluid, modulated the production of pro-inflammatory and anti-inflammatory cytokines in the splenocytes and lungs of allergic mice, reduced the percentage of Th2 cells and increased the proportion of Th1 and regulatory T cells (Tregs). At the same time, Foxp3 and T-bet expression increased, and GATA3 and RORγt decreased in the lungs of allergic mice. We proved that SJMHE1 can interrupt the development of asthma by diminishing airway inflammation in mice. The down-regulation of Th2 response and the up-regulation of Th1 and Tregs response may contribute to the protection induced by SJMHE1 in allergic mice. SJMHE1 can serve as a novel therapy for asthma and other allergic or inflammatory diseases.
Keywords: SJMHE1; Schistosoma japonicum peptide; airway inflammation; allergic asthma; suppress.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors declare that they have no competing interests. The funding agencies played no role in the design or implementation of the study, analysis or interpretation of the data, or the preparation and submission of the manuscript.
Figures

References
-
- Shi YH, Shi GC, Wan HY, et al. Coexistence of Th1/Th2 and Th17/Treg imbalances in patients with allergic asthma. Chin Med J. 2011;124:1951‐1956. - PubMed
-
- Gilchrist FJ, Ahmad AN, Batchelor HK, Marriott JF, Lenney W. A review of prednisolone prescribing for children with acute asthma in the UK. J Asthma. 2016;53:563‐566. - PubMed
-
- Choby GW, Lee S. Pharmacotherapy for the treatment of asthma: current treatment options and future directions. Int Forum Allergy Rhinol. 2015;5(Suppl 1):S35‐40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

