Reversal of epigenetic aging and immunosenescent trends in humans
- PMID: 31496122
- PMCID: PMC6826138
- DOI: 10.1111/acel.13028
Reversal of epigenetic aging and immunosenescent trends in humans
Abstract
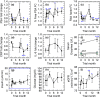
Epigenetic "clocks" can now surpass chronological age in accuracy for estimating biological age. Here, we use four such age estimators to show that epigenetic aging can be reversed in humans. Using a protocol intended to regenerate the thymus, we observed protective immunological changes, improved risk indices for many age-related diseases, and a mean epigenetic age approximately 1.5 years less than baseline after 1 year of treatment (-2.5-year change compared to no treatment at the end of the study). The rate of epigenetic aging reversal relative to chronological age accelerated from -1.6 year/year from 0-9 month to -6.5 year/year from 9-12 month. The GrimAge predictor of human morbidity and mortality showed a 2-year decrease in epigenetic vs. chronological age that persisted six months after discontinuing treatment. This is to our knowledge the first report of an increase, based on an epigenetic age estimator, in predicted human lifespan by means of a currently accessible aging intervention.
Keywords: PD-1; PSA; c-reactive protein; lymphocyte-to-monocyte ratio; naive T cells; thymic regeneration.
© 2019 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
GMF, RTB, JPW, and SH are shareholders in or have options to purchase shares in Intervene Immune, Inc., GMF and RTB are officers of Intervene Immune and are named in a related Intervene Immune patent application. All other authors declare no competing interests.
Figures




Comment in
-
First hint that body's 'biological age' can be reversed.Nature. 2019 Sep;573(7773):173. doi: 10.1038/d41586-019-02638-w. Nature. 2019. PMID: 31506619 No abstract available.
References
-
- Arnold, C. , Wolf, J. , Brunner, S. , Herndler‐Brandstetter, D. , & Grubeck‐Loebenstein, B. (2011). Gain and loss of T cell subsets in old age – age‐associated reshaping of the T cell repertoire. Journal of Clinical Immunology, 31, 137–146. - PubMed
-
- Bodey, B. , Bodey, B. J. , Siegel, S. E. , & Kaiser, H. E. (1997). Involution of the mammalian thymus, one of the leading regulators of aging. In Vivo, 11, 421–440. - PubMed
-
- Caglayan, V. , Onen, E. , Avci, S. , Sambel, M. , Kilic, M. , … Yidiz, H. E. (2019). Lymphocyte‐to‐monocyte ratio is a valuable marker to predict cancer in patients with prostate specific antigen between 4 and 10 ng/dl. Archivio Italiano Di Urologia, Andrologia, 90, 270–275. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

