Hypoxic Isolated Abdominal Perfusion (HAP) chemotherapy for non-operable advanced staged ovarian cancer with peritoneal carcinosis: an experience in 45 platinum-refractory ovarian cancer patients
- PMID: 31496601
- PMCID: PMC6707993
- DOI: 10.1007/s13193-019-00922-9
Hypoxic Isolated Abdominal Perfusion (HAP) chemotherapy for non-operable advanced staged ovarian cancer with peritoneal carcinosis: an experience in 45 platinum-refractory ovarian cancer patients
Abstract
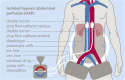
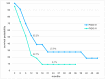
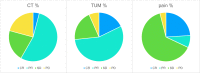
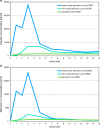
In order to break through drug resistance in platinum-refractory ovarian cancer, augmented drug exposure was administered to the abdomen by means of an isolated perfusion system. Four cycles of isolated hypoxic abdominal perfusion with cisplatin, adriamycin, and mitomycin were conducted in 4-week intervals. Cisplatin and adriamycin were chosen because of their increased cytotoxicity under hypoxic conditions. Chemofiltration was performed for prophylaxis of cumulative toxicity of adriamycin and mitomycin. The study included 45 patients with recurrent epithelial ovarian cancer who had prior platinum containing therapies (3, stage Federation of Gynecology and Obstetrics (FIGO) IIIB; 20, stage FIGO IIIC; 22; stage FIGO IV). The median survival rate in stage FIGO IIIBC was 12 months, and in stage IV was 10 months. The tumor marker decreased to complete response or partial response at 17.8% and 55.6% of the patients. CT or MRI visualization showed complete response in 4.1%, and partial response was in 54.1%. Complete resolution of ascites was noted in 30% of cases and substantial reduction in another 43%. Toxicity was generally low. Quality of life was improved in the majority of cases. Bone-marrow suppression ranged between WHO grade 1 and 2, and in patients with previous third- or fourth-line chemotherapy, it was WHO grade 3. Isolated hypoxic abdominal perfusion with chemofiltration for patients with progressive and platinum-refractory stage III and IV ovarian cancer is an effective therapy, breaking through chemoresistance and offering comparably long survival at good quality of life.
Keywords: Chemoresistance; Intra-arterial chemotherapy; Isolated abdominal perfusion; Ovarian cancer; Quality of life.
Conflict of interest statement
Conflict of InterestThe authors declare that they have no conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
