Methionine adenosyltransferases in liver cancer
- PMID: 31496615
- PMCID: PMC6710175
- DOI: 10.3748/wjg.v25.i31.4300
Methionine adenosyltransferases in liver cancer
Abstract
Methionine adenosyltransferases (MATs) are essential enzymes for life as they produce S-adenosylmethionine (SAMe), the biological methyl donor required for a plethora of reactions within the cell. Mammalian systems express two genes, MAT1A and MAT2A, which encode for MATα1 and MATα2, the catalytic subunits of the MAT isoenzymes, respectively. A third gene MAT2B, encodes a regulatory subunit known as MATβ which controls the activity of MATα2. MAT1A, which is mainly expressed in hepatocytes, maintains the differentiated state of these cells, whilst MAT2A and MAT2B are expressed in extrahepatic tissues as well as non-parenchymal cells of the liver (e.g., hepatic stellate and Kupffer cells). The biosynthesis of SAMe is impaired in patients with chronic liver disease and liver cancer due to decreased expression and inactivation of MATα1. A switch from MAT1A to MAT2A/MAT2B occurs in multiple liver diseases and during liver growth and dedifferentiation, but this change in the expression pattern of MATs results in reduced hepatic SAMe level. Decades of study have utilized the Mat1a-knockout (KO) mouse that spontaneously develops non-alcoholic steatohepatitis (NASH) and hepatocellular carcinoma (HCC) to elucidate a variety of mechanisms by which MAT proteins dysregulation contributes to liver carcinogenesis. An increasing volume of work indicates that MATs have SAMe-independent functions, distinct interactomes and multiple subcellular localizations. Here we aim to provide an overview of MAT biology including genes, isoenzymes and their regulation to provide the context for understanding consequences of their dysregulation. We will highlight recent breakthroughs in the field and underscore the importance of MAT's in liver tumorigenesis as well as their potential as targets for cancer therapy.
Keywords: Biomarkers; Cholangiocarcinoma; Hepatocellular carcinoma; Liver cancer; Methionine adenosyltransferases; S-adenosylmethionine; Therapeutic targets.
Conflict of interest statement
Conflict-of-interest statement: None of the authors have any conflict of interest.
Figures
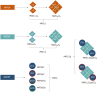

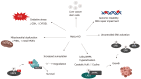

Similar articles
-
Methionine adenosyltransferases in cancers: Mechanisms of dysregulation and implications for therapy.Exp Biol Med (Maywood). 2018 Jan;243(2):107-117. doi: 10.1177/1535370217740860. Epub 2017 Nov 15. Exp Biol Med (Maywood). 2018. PMID: 29141455 Free PMC article. Review.
-
Pleiotropic effects of methionine adenosyltransferases deregulation as determinants of liver cancer progression and prognosis.J Hepatol. 2013 Oct;59(4):830-41. doi: 10.1016/j.jhep.2013.04.031. Epub 2013 May 7. J Hepatol. 2013. PMID: 23665184 Review.
-
Role of methionine adenosyltransferase and S-adenosylmethionine in alcohol-associated liver cancer.Alcohol. 2005 Apr;35(3):227-34. doi: 10.1016/j.alcohol.2005.03.011. Alcohol. 2005. PMID: 16054984 Review.
-
Methionine adenosyltransferases in liver health and diseases.Liver Res. 2017 Sep;1(2):103-111. doi: 10.1016/j.livres.2017.07.002. Liver Res. 2017. PMID: 29170720 Free PMC article.
-
The Oncogene PDRG1 Is an Interaction Target of Methionine Adenosyltransferases.PLoS One. 2016 Aug 22;11(8):e0161672. doi: 10.1371/journal.pone.0161672. eCollection 2016. PLoS One. 2016. PMID: 27548429 Free PMC article.
Cited by
-
Prohibitin 1 in liver injury and cancer.Exp Biol Med (Maywood). 2020 Mar;245(5):385-394. doi: 10.1177/1535370220908257. Epub 2020 Feb 20. Exp Biol Med (Maywood). 2020. PMID: 32077311 Free PMC article. Review.
-
SYVN1-MTR4-MAT2A Signaling Axis Regulates Methionine Metabolism in Glioma Cells.Front Cell Dev Biol. 2021 Mar 30;9:633259. doi: 10.3389/fcell.2021.633259. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33859984 Free PMC article.
-
S-adenosylmethionine synthases specify distinct H3K4me3 populations and gene expression patterns during heat stress.Elife. 2023 Feb 9;12:e79511. doi: 10.7554/eLife.79511. Elife. 2023. PMID: 36756948 Free PMC article.
-
Structure-activity features of purines and their receptors: implications in cell physiopathology.Mol Biomed. 2022 Jan 26;3(1):5. doi: 10.1186/s43556-022-00068-1. Mol Biomed. 2022. PMID: 35079944 Free PMC article. Review.
-
A New Proton Transfer Complex Between 3,4-Diaminopyridine Drug and 2,6-Dichloro-4-nitrophenol: Synthesis, Spectroscopic Characterization, DFT Studies, DNA Binding Analysis, and Antitumor Activity.Molecules. 2024 Oct 30;29(21):5120. doi: 10.3390/molecules29215120. Molecules. 2024. PMID: 39519761 Free PMC article.
References
-
- Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM, Anderson RN, Ma J, Ly KN, Cronin KA, Penberthy L, Kohler BA. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–1337. - PMC - PubMed
-
- Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. - PubMed
-
- Diehl AM, Day C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N Engl J Med. 2017;377:2063–2072. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

