Eicosanoids metabolized through LOX distinguish asthma-COPD overlap from COPD by metabolomics study
- PMID: 31496676
- PMCID: PMC6689553
- DOI: 10.2147/COPD.S207023
Eicosanoids metabolized through LOX distinguish asthma-COPD overlap from COPD by metabolomics study
Abstract
Background and objective: The prevalence of asthma is greater than 20% in patients previously diagnosed with COPD. Patients with asthma-COPD overlap (ACO) are at risk of rapid progression of disease and severe exacerbations. However, in some patients with ACO, a clear distinction from COPD is very difficult by using physiological testing techniques. This study aimed to apply a novel metabolomic approach to identify the metabolites in sera in order to distinguish ACO from COPD.
Methods: In the study, blood samples were collected from patients with COPD, ACO, and healthy controls. Cholamine derivatization-ultrahigh performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UHPLC-Q-TOF/MS) was used to investigate serum metabolites of eicosanoids.
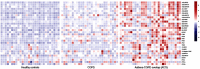
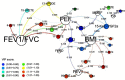
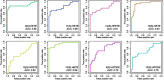
Results: A clear intergroup separation existed between the patients with ACO and those with COPD, while ACO tends to have higher serum metabolic levels of eicosanoids. A robust Orthogonal Projections to Latent Structures-Discriminant Analysis (OPLS-DA) model was found for discriminating between ACO and COPD (R2Y =0.81, Q2=0.79). In addition, there is a significant correlation between some metabolites and clinical indicators, such as hydroxyeicosatetraenoic acids (HETEs), hydroperoxyeicosatetraenoic acids (HPETEs) and FEV1/FVC. The higher values of area under the receiver operating characteristic curves (ROC) of HETEs, which were metabolized from HPETEs through lipoxygenase (LOX), indicated that they should be the potential biomarkers to distinguish ACO from COPD.
Conclusion: Eicosanoids can clearly discriminate different biochemical metabolic profiles between ACO and COPD. The results possibly provide a new perspective to identify potential biomarkers of ACO and may be helpful for personalized treatment.
Keywords: COPD; cholamine derivatization-UHPLC-Q-TOF/MS; metabolomics; asthma–COPD overlap.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures