Effect of tiotropium/olodaterol on sedentary and active time in patients with COPD: post hoc analysis of the VESUTO® study
- PMID: 31496678
- PMCID: PMC6689763
- DOI: 10.2147/COPD.S208081
Effect of tiotropium/olodaterol on sedentary and active time in patients with COPD: post hoc analysis of the VESUTO® study
Erratum in
-
Erratum: Effect of tiotropium/olodaterol on sedentary and active time in patients with COPD: post hoc analysis of the VESUTO® study [Erratum].Int J Chron Obstruct Pulmon Dis. 2019 Sep 3;14:2061-2064. doi: 10.2147/COPD.S229124. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 31564853 Free PMC article.
Abstract
Background: Patients with COPD are less physically active. This post hoc analysis of a randomized, double-blind, active-controlled, crossover trial assessed the efficacy of once-daily tiotropium/olodaterol combination therapy versus tiotropium monotherapy in Japanese patients with COPD.
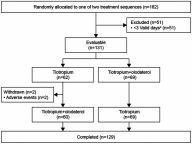
Patients and methods: Patients were provided with a three-axis accelerometer to measure sedentary and active behavior defined as 1.0-1.5 metabolic equivalents (METs), ≥2.0 METs, and ≥3.0 METs, respectively. Of the 182 patients enrolled, 131 satisfied the conditions for the present analysis and were randomized to tiotropium monotherapy (n=62) or tiotropium/olodaterol combination therapy (n=69).
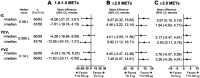
Results: Tiotropium/olodaterol combination therapy significantly reduced the duration of 1.0-1.5 MET activity by 8.64 mins (p=0.040) and significantly increased the duration of ≥2.0 MET and ≥3.0 MET activity by 6.51 mins (p=0.017) and 2.60 mins (p=0.008), respectively, compared with tiotropium alone. Subgroup analyses showed that better lung function, milder dyspnea, and higher levels of physical activity at baseline were associated with reduced sedentary time and increased duration of physical activity.
Conclusion: Tiotropium/olodaterol combination therapy significantly reduced sedentary time and improved physical activity compared with tiotropium monotherapy. This trial was registered in ClinicalTrials.gov (NCT02629965).
Keywords: COPD; Japanese; physical activity; sedentary behavior.
Conflict of interest statement
Y. Minakata reports non financial support from Nippon Boehringer Ingelheim, during the conduct of the study and personal fees from Nippon Boehringer Ingelheim, outside the submitted work. T. Motegi reports personal fees, non financial support from Nippon Boehringer Ingelheim, during the conduct of the study; personal fees from Nippon Boehringer Ingelheim, Fukuda Life Tech and AstraZeneca, outside the submitted work. J. Ueki reports personal fees from Nippon Boehringer Ingelheim, Hoshi Iryo Sanki, Teijin Pharma and Novartis Pharma, during the conduct of the study. Y. Gon reports personal fees from Nippon Boehringer Ingelheim, during the conduct of the study and personal fees from Novartis Pharma, Nippon Boehringer Ingelheim, Astra Zeneca and Kyorin Pharma, outside the submitted work. S. Nakamura is an employee of Nippon Boehringer Ingelheim. T. Anzai reports compensation for statistical analysis services from Nippon Boehringer Ingelheim during the conduct of the study and outside the submitted work. K. Hirata reports non financial support from Nippon Boehringer Ingelheim, during the conduct of the study and personal fees from Nippon Boehringer Ingelheim, outside the submitted work. M. Ichinose has received honoraria from AstraZeneca, Nippon Boehringer Ingelheim, and Novartis Pharma. The authors report no other conflicts of interest in this work.
Figures


Similar articles
-
Factors Associated with Reduction of Sedentary Time Following Tiotropium/Olodaterol Therapy in Treatment-Naïve Chronic Obstructive Pulmonary Disease.Int J Chron Obstruct Pulmon Dis. 2021 Dec 6;16:3297-3307. doi: 10.2147/COPD.S338560. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 34908832 Free PMC article. Clinical Trial.
-
Study Design of VESUTO®: Efficacy of Tiotropium/Olodaterol on Lung Hyperinflation, Exercise Capacity, and Physical Activity in Japanese Patients with Chronic Obstructive Pulmonary Disease.Adv Ther. 2017 Jul;34(7):1622-1635. doi: 10.1007/s12325-017-0554-3. Epub 2017 May 23. Adv Ther. 2017. PMID: 28537001 Free PMC article.
-
The lung function profile of once-daily tiotropium and olodaterol via Respimat(®) is superior to that of twice-daily salmeterol and fluticasone propionate via Accuhaler(®) (ENERGITO(®) study).Int J Chron Obstruct Pulmon Dis. 2016 Feb 4;11:193-205. doi: 10.2147/COPD.S95055. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 26893551 Free PMC article. Clinical Trial.
-
Tiotropium/olodaterol (Stiolto Respimat) for COPD.Med Lett Drugs Ther. 2015 Nov 23;57(1482):161-2. Med Lett Drugs Ther. 2015. PMID: 26583607 Review. No abstract available.
-
Olodaterol + tiotropium bromide for the treatment of chronic obstructive pulmonary disease.Expert Rev Clin Pharmacol. 2015;8(5):529-39. doi: 10.1586/17512433.2015.1075389. Expert Rev Clin Pharmacol. 2015. PMID: 26294073 Review.
Cited by
-
Factors Associated with Reduction of Sedentary Time Following Tiotropium/Olodaterol Therapy in Treatment-Naïve Chronic Obstructive Pulmonary Disease.Int J Chron Obstruct Pulmon Dis. 2021 Dec 6;16:3297-3307. doi: 10.2147/COPD.S338560. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 34908832 Free PMC article. Clinical Trial.
-
A randomized controlled trial of long-acting muscarinic antagonist and long-acting β2 agonist fixed-dose combinations in patients with chronic obstructive pulmonary disease.BMC Pulm Med. 2021 Jan 13;21(1):26. doi: 10.1186/s12890-021-01403-y. BMC Pulm Med. 2021. PMID: 33441146 Free PMC article. Clinical Trial.
-
LABA/LAMA as First-Line Therapy for COPD: A Summary of the Evidence and Guideline Recommendations.J Clin Med. 2022 Nov 8;11(22):6623. doi: 10.3390/jcm11226623. J Clin Med. 2022. PMID: 36431099 Free PMC article. Review.
-
Data Reproducibility and Effectiveness of Bronchodilators for Improving Physical Activity in COPD Patients.J Clin Med. 2020 Oct 29;9(11):3497. doi: 10.3390/jcm9113497. J Clin Med. 2020. PMID: 33138116 Free PMC article. Review.
-
Future concepts in bronchodilation for COPD: dual- versus monotherapy.Eur Respir Rev. 2021 Jun 1;30(160):210023. doi: 10.1183/16000617.0023-2021. Print 2021 Jun 30. Eur Respir Rev. 2021. PMID: 34415847 Free PMC article. Review.
References
-
- Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–S504. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

