Mechano-signalling, induced by fullerene C60 nanofilms, arrests the cell cycle in the G2/M phase and decreases proliferation of liver cancer cells
- PMID: 31496681
- PMCID: PMC6689765
- DOI: 10.2147/IJN.S206934
Mechano-signalling, induced by fullerene C60 nanofilms, arrests the cell cycle in the G2/M phase and decreases proliferation of liver cancer cells
Abstract
Introduction and objective: Degradation of the extracellular matrix (ECM) changes the physicochemical properties and dysregulates ECM-cell interactions, leading to several pathological conditions, such as invasive cancer. Carbon nanofilm, as a biocompatible and easy to functionalize material, could be used to mimic ECM structures, changing cancer cell behavior to perform like normal cells.
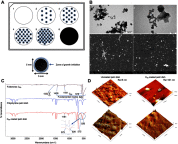
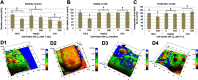
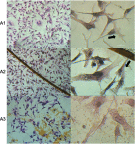
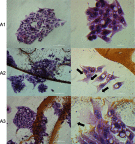
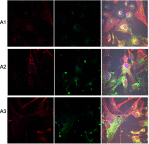
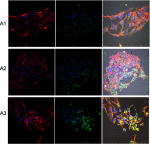
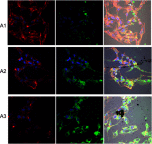
Methods: Experiments were performed in vitro with HS-5 cells (as a control) and HepG2 and C3A cancer cells. An aqueous solution of fullerene C60 was used to form a nanofilm. The morphological properties of cells cultivated on C60 nanofilms were evaluated with light, confocal, electron and atomic force microscopy. The cell viability and proliferation were measured by XTT and BrdU assays. Immunoblotting and flow cytometry were used to evaluate the expression level of proliferating cell nuclear antigen and determine the number of cells in the G2/M phase.
Results: All cell lines were spread on C60 nanofilms, showing a high affinity to the nanofilm surface. We found that C60 nanofilm mimicked the niche/ECM of cells, was biocompatible and non-toxic, but the mechanical signal from C60 nanofilm created an environment that affected the cell cycle and reduced cell proliferation.
Conclusion: The results indicate that C60 nanofilms might be a suitable, substitute component for the niche of cancer cells. The incorporation of fullerene C60 in the ECM/niche may be an alternative treatment for hepatocellular carcinoma.
Keywords: adhesion; cell cycle; extracellular matrix; fullerene; liver cancer cells.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures





Similar articles
-
Polyhydroxylated Fullerene C60(OH)40 Nanofilms Promote the Mesenchymal-Epithelial Transition of Human Liver Cancer Cells via the TGF-β1/Smad Pathway.J Inflamm Res. 2023 Aug 29;16:3739-3761. doi: 10.2147/JIR.S415378. eCollection 2023. J Inflamm Res. 2023. PMID: 37663761 Free PMC article.
-
Diamond Nanofilm Normalizes Proliferation and Metabolism in Liver Cancer Cells.Nanotechnol Sci Appl. 2021 Aug 28;14:115-137. doi: 10.2147/NSA.S322766. eCollection 2021. Nanotechnol Sci Appl. 2021. PMID: 34511890 Free PMC article.
-
Growth and potential damage of human bone-derived cells on fresh and aged fullerene c60 films.Int J Mol Sci. 2013 Apr 26;14(5):9182-204. doi: 10.3390/ijms14059182. Int J Mol Sci. 2013. PMID: 23624607 Free PMC article.
-
[Preparation and Evaluation of Fullerene Based Nanomedicine].Yakugaku Zasshi. 2019;139(12):1539-1546. doi: 10.1248/yakushi.19-00172. Yakugaku Zasshi. 2019. PMID: 31787641 Review. Japanese.
-
The Extracellular Matrix and Biocompatible Materials in Glioblastoma Treatment.Front Bioeng Biotechnol. 2019 Nov 19;7:341. doi: 10.3389/fbioe.2019.00341. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31803736 Free PMC article. Review.
Cited by
-
Graphene Oxide Scaffold Stimulates Differentiation and Proangiogenic Activities of Myogenic Progenitor Cells.Int J Mol Sci. 2020 Jun 11;21(11):4173. doi: 10.3390/ijms21114173. Int J Mol Sci. 2020. PMID: 32545308 Free PMC article.
-
High Expression of PIGC Predicts Unfavorable Survival in Hepatocellular Carcinoma.J Hepatocell Carcinoma. 2021 Apr 6;8:211-222. doi: 10.2147/JHC.S297601. eCollection 2021. J Hepatocell Carcinoma. 2021. PMID: 33854986 Free PMC article.
-
Extracellular matrix remodeling in tumor progression and immune escape: from mechanisms to treatments.Mol Cancer. 2023 Mar 11;22(1):48. doi: 10.1186/s12943-023-01744-8. Mol Cancer. 2023. PMID: 36906534 Free PMC article. Review.
-
Reduced Graphene Oxide Modulates the FAK-Dependent Signaling Pathway in Glioblastoma Multiforme Cells In Vitro.Materials (Basel). 2022 Aug 24;15(17):5843. doi: 10.3390/ma15175843. Materials (Basel). 2022. PMID: 36079225 Free PMC article.
-
Versatile carbon nanoplatforms for cancer treatment and diagnosis: strategies, applications and future perspectives.Theranostics. 2022 Feb 21;12(5):2290-2321. doi: 10.7150/thno.69628. eCollection 2022. Theranostics. 2022. PMID: 35265211 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

