Synthesis and characterization of a long-acting emtricitabine prodrug nanoformulation
- PMID: 31496683
- PMCID: PMC6689761
- DOI: 10.2147/IJN.S215447
Synthesis and characterization of a long-acting emtricitabine prodrug nanoformulation
Abstract
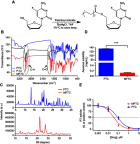
Purpose: A palmitoylated prodrug of emtricitabine (FTC) was synthesized to extend the drug's half-life, antiretroviral activities and biodistribution.
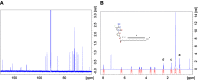
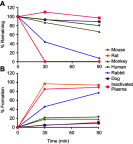
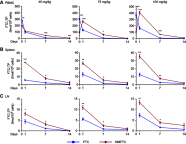
Methods: A modified FTC prodrug (MFTC) was synthesized by palmitoyl chloride esterification. MFTC's chemical structure was evaluated by nuclear magnetic resonance. The created hydrophobic prodrug nanocrystals were encased into a poloxamer surfactant and the pharmacokinetics (PK), biodistribution and antiretroviral activities of the nanoformulation (NMFTC) were assessed. The conversion of MFTC to FTC triphosphates was evaluated.
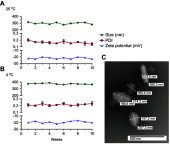
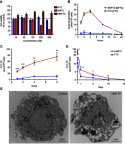
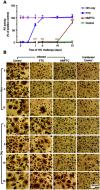
Results: MFTC coated with poloxamer formed stable nanocrystals (NMFTC). NMFTC demonstrated an average particle size, polydispersity index and zeta potential of 350 nm, 0.24 and -20 mV, respectively. Drug encapsulation efficiency was 90%. NMFTC was readily taken up by human monocyte-derived macrophages yielding readily detected intracellular FTC triphosphates and an extended PK profile.
Conclusion: NMFTC shows improved antiretroviral activities over native FTC. This is coordinate with its extended apparent half-life. The work represents an incremental advance in the development of a long-acting FTC formulation.
Keywords: monocyte-derived macrophage; human immunodeficiency virus type 1; long-acting antiretrovirals; palmitoyl chloride; viral reservoirs.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

References
-
- Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15(9):1181–1183. - PubMed
MeSH terms
Substances
Grants and funding
- R01 NS036126/NS/NINDS NIH HHS/United States
- R01 MH115860/MH/NIMH NIH HHS/United States
- R01 AI145542/AI/NIAID NIH HHS/United States
- P01 DA028555/DA/NIDA NIH HHS/United States
- P30 MH062261/MH/NIMH NIH HHS/United States
- P01 NS031492/NS/NINDS NIH HHS/United States
- R56 AI138613/AI/NIAID NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- R01 MH104147/MH/NIMH NIH HHS/United States
- R01 AG043540/AG/NIA NIH HHS/United States
- P30 AI078498/AI/NIAID NIH HHS/United States
- R01 NS034239/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

