Intravesical delivery of rapamycin via folate-modified liposomes dispersed in thermo-reversible hydrogel
- PMID: 31496684
- PMCID: PMC6689153
- DOI: 10.2147/IJN.S216432
Intravesical delivery of rapamycin via folate-modified liposomes dispersed in thermo-reversible hydrogel
Abstract
Purpose: To develop an intravesical instillation system for the treatment of bladder cancer, rapamycin (Rap) was encapsulated into liposomes and then homogeneously dispersed throughout a poloxamer 407 (P407)-based hydrogel.
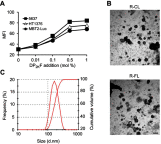
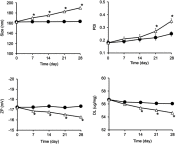
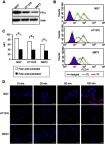
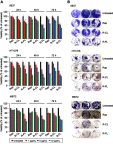
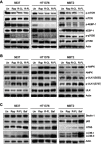
Methods: Rap-loaded conventional liposomes (R-CL) and folate-modified liposomes (R-FL) were prepared using a film hydration method and pre-loading technique, and characterized by particle size, drug entrapment efficiency, and drug loading. The cellular uptake behavior in folate receptor-expressing bladder cancer cells was observed by flow cytometry and confocal laser scanning microscopy using a fluorescent probe. In vitro cytotoxic effects were evaluated using MTT assay, colony forming assay, and Western blot. For in vivo intravesical instillation, Rap-loaded liposomes were dispersed in P407-gel, generating R-CL/P407 and R-FL/P407. Gel-forming capacities and drug release were evaluated. Using the MBT2/Luc orthotopic bladder cancer mouse model, in vivo antitumor efficacy was evaluated according to regions of interest (ROI) measurement.
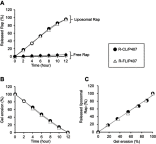
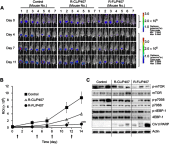
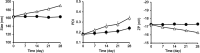
Results: R-CL and R-FL were successfully prepared, at approximately <160 nm, 42% entrapment efficiency, and 57 μg/mg drug loading. FL cellular uptake was enhanced over 2-fold than that of CL; folate receptor-mediated endocytosis was confirmed using a competitive assay with folic acid pretreatment. In vitro cytotoxic effects increased dose-dependently. Rap-loaded liposomes inhibited mTOR signaling and induced autophagy in urothelial carcinoma cells. With gelation time of <30 seconds and gel duration of >12 hrs, both R-CL/P407 and R-FL/P407 preparations transformed into gel immediately after instillation into the mouse bladder. Drug release from the liposomal gel was erosion controlled. In orthotopic bladder cancer mouse model, statistically significant differences in ROI values were found between R-CL/P407 and R-FL/P407 groups at day 11 (P=0.0273) and day 14 (P=0.0088), indicating the highest tumor growth inhibition by R-FL/P407.
Conclusion: Intravesical instillation of R-FL/P407 might represent a good candidate for bladder cancer treatment, owing to its enhanced retention and FR-targeting.
Keywords: antitumor efficacy; autophagy; bladder cancer; enhanced uptake; mTOR signaling; prolonged retention.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
A floating hydrogel system capable of generating CO2 bubbles to diminish urinary obstruction after intravesical instillation.Pharm Res. 2014 Oct;31(10):2655-63. doi: 10.1007/s11095-014-1362-y. Epub 2014 Apr 22. Pharm Res. 2014. PMID: 24752478
-
Gemcitabine hydrochloride microspheres used for intravesical treatment of superficial bladder cancer: a comprehensive in vitro/ex vivo/in vivo evaluation.Drug Des Devel Ther. 2018 Jul 2;12:1959-1975. doi: 10.2147/DDDT.S164704. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 29997433 Free PMC article.
-
Folate receptor-targeted liposomes enhanced the antitumor potency of imatinib through the combination of active targeting and molecular targeting.Int J Nanomedicine. 2014 May 7;9:2167-78. doi: 10.2147/IJN.S60178. eCollection 2014. Int J Nanomedicine. 2014. PMID: 24855354 Free PMC article.
-
Drug-Loaded Biocompatible Nanocarriers Embedded in Poloxamer 407 Hydrogels as Therapeutic Formulations.Medicines (Basel). 2018 Dec 29;6(1):7. doi: 10.3390/medicines6010007. Medicines (Basel). 2018. PMID: 30597953 Free PMC article. Review.
-
Recent advances in intravesical drug/gene delivery.Mol Pharm. 2006 Jul-Aug;3(4):369-79. doi: 10.1021/mp060001j. Mol Pharm. 2006. PMID: 16889430 Free PMC article. Review.
Cited by
-
Nanomedicine Approaches for Autophagy Modulation in Cancer Therapy.Small Sci. 2025 Apr 11;5(6):2400607. doi: 10.1002/smsc.202400607. eCollection 2025 Jun. Small Sci. 2025. PMID: 40529859 Free PMC article.
-
Low-dose versus standard-dose bacille Calmette-Guérin for non-muscle-invasive bladder cancer: Systematic review and meta-analysis of randomized controlled trials.Investig Clin Urol. 2022 Mar;63(2):140-150. doi: 10.4111/icu.20210340. Investig Clin Urol. 2022. PMID: 35244987 Free PMC article.
-
Delivery of Rapamycin by Liposomes Synergistically Enhances the Chemotherapy Effect of 5-Fluorouracil on Colorectal Cancer.Int J Nanomedicine. 2021 Jan 12;16:269-281. doi: 10.2147/IJN.S270939. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33469286 Free PMC article.
-
Progress on liposome delivery systems in the treatment of bladder cancer.RSC Adv. 2025 May 6;15(18):14315-14336. doi: 10.1039/d5ra00746a. eCollection 2025 Apr 28. RSC Adv. 2025. PMID: 40330044 Free PMC article. Review.
-
Design and Evaluation of a Dual-Sensitive In Situ Gel for the Controlled Release of Pranoprofen.AAPS PharmSciTech. 2024 Feb 8;25(2):35. doi: 10.1208/s12249-024-02748-3. AAPS PharmSciTech. 2024. PMID: 38332223
References
-
- Jensen TK, Holt P, Gerke O, et al. Preoperative lymph-node staging of invasive urothelial bladder cancer with 18F-fluorodeoxyglucose positron emission tomography/computed axial tomography and magnetic resonance imaging: correlation with histopathology. Scand J Urol Nephrol. 2011;45:122–128. doi:10.3109/00365599.2010.544672 - DOI - PubMed
-
- Mattioli F, Curotto A, Manfredi V, et al. Intravesical gemcitabine in superficial bladder cancer: a phase II safety, efficacy and pharmacokinetic study. Anticancer Res. 2005;25:2493–2496. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

