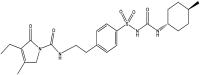
Fabrication and characterization of glimepiride nanosuspension by ultrasonication-assisted precipitation for improvement of oral bioavailability and in vitro α-glucosidase inhibition
- PMID: 31496686
- PMCID: PMC6689535
- DOI: 10.2147/IJN.S210548
Fabrication and characterization of glimepiride nanosuspension by ultrasonication-assisted precipitation for improvement of oral bioavailability and in vitro α-glucosidase inhibition
Erratum in
-
Erratum: Fabrication and Characterization of Glimepiride Nanosuspension by Ultrasonication-Assisted Precipitation for Improvement of Oral Bioavailability and in vitro α-Glucosidase Inhibition [Corrigendum].Int J Nanomedicine. 2020 Sep 4;15:6561-6562. doi: 10.2147/IJN.S279083. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32982217 Free PMC article.
Retraction in
-
Fabrication and Characterization of Glimepiride Nanosuspension by Ultrasonication-Assisted Precipitation for Improvement of Oral Bioavailability and in vitro α-Glucosidase Inhibition [Retraction].Int J Nanomedicine. 2022 Nov 28;17:5779-5780. doi: 10.2147/IJN.S398841. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36466786 Free PMC article.
Abstract
Purpose: We aimed to enhance the solubility, dissolution rate, oral bioavailability, and α-glucosidase inhibition of glimepiride (Glm) by fabricating its nanosuspension using a precipitation-ultrasonication approach.
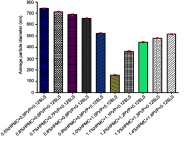
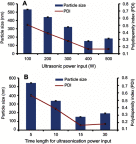
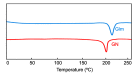
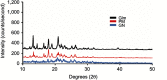
Methods: Glm nanosuspensions were fabricated using optimized processing conditions. Characterization of Glm was performed using Malvern Zetasizer, scanning electron microscopy, transmission electron microscopy, differential scanning calorimetry, and powder X-ray diffraction. Minimum particle size and polydispersity index (PDI) values were found to be 152.4±2.42 nm and 0.23±0.01, respectively, using hydroxypropyl methylcellulose: 6 cPs, 1% w/v, polyvinylpyrrolidone K30 1% w/v, and sodium lauryl sulfate 0.12% w/v, keeping ultrasonication power input at 400 W, with 15 minutes' processing at 3-second pauses. In vivo oral bioavailability was assessed using rabbits as a model.
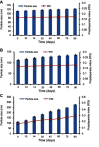
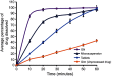
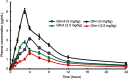
Results: The saturation solubility of the Glm nanosuspensions was substantially enhanced 3.14-fold and 5.77-fold compared to unprocessed drug in stabilizer solution and unprocessed active pharmaceutical ingredient. Also, the dissolution rate of the nanosuspensions ws substantially boosted when compared to the marketed formulation and unprocessed drug candidate. The results showed that >85% of Glm nanosuspensions dissolved in the first 10 minutes compared to 10.17% of unprocessed Glm), 42.19% of microsuspensions, and 19.94% of marketed tablets. In-vivo studies conducted in animals, i.e. rabbits, demonstrated that maximum concentration and AUC0-24 with oral dosing were twofold (5 mg/kg) and 1.74-fold (2.5 mg/kg) and 1.80-fold (5 mg/kg) and 1.63-fold (2.5 mg/kg), respectively, and compared with the unprocessed drug formulation. In-vitro α-glucosidase inhibition results showed that fabricated nanosuspensions had a pronounced effect compared to unprocessed drug.
Conclusion: The optimized batch fabricated by ultrasonication-assisted precipitation can be useful in boosting oral bioavailability, which may be accredited to enhanced solubility and dissolution rate of Glm, ultimately resulting in its faster rate of absorption due to nanonization.
Keywords: boosted bioavailability; glimepiride nanosuspension; precipitation–ultrasonication approach.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
-
- Sahu BP, Das MK. Nanosuspension for enhancement of oral bioavailability of felodipine. Appl Nanosci. 2014;4(2):189–197. doi: 10.1007/s13204-012-0188-3 - DOI
-
- Reverchon E. Supercritical antisolvent precipitation of micro-and nano-particles. J Supercrit Fluids. 1999;15(1):1–21. doi: 10.1016/S0896-8446(98)00129-6 - DOI
-
- Pathak P, Meziani MJ, Desai T, Sun Y-P. Formation and stabilization of ibuprofen nanoparticles in supercritical fluid processing. J Supercrit Fluids. 2006;37(3):279–286. doi: 10.1016/j.supflu.2005.09.005 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

