Titania nanotube-based protein delivery system to inhibit cranial bone regeneration in Crouzon model of craniosynostosis
- PMID: 31496688
- PMCID: PMC6690047
- DOI: 10.2147/IJN.S202090
Titania nanotube-based protein delivery system to inhibit cranial bone regeneration in Crouzon model of craniosynostosis
Abstract
Background: Craniosynostosis is a developmental disorder characterized by the premature fusion of skull sutures, necessitating repetitive, high-risk neurosurgical interventions throughout infancy. This study used protein-releasing Titania nanotubular implant (TNT/Ti) loaded with glypican 3 (GPC3) in the cranial critical-sized defects (CSDs) in Crouzon murine model (Fgfr2c342y/+ knock-in mutation) to address a key challenge of delaying post-operative bone regeneration in craniosynostosis.
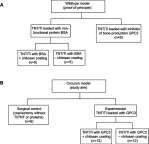
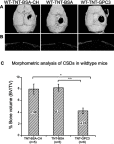
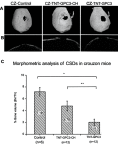
Materials and methods: A 3 mm wide circular CSD was created in two murine models of Crouzon syndrome: (i) surgical control (CSDs without TNT/Ti or any protein, n=6) and (ii) experimental groups with TNT/Ti loaded with GPC3, further subdivided into the presence or absence of chitosan coating (on nanotubes) (n=12 in each group). The bone volume percentage in CSDs was assessed 90 days post-implantation using micro-computed tomography (micro-CT) and histological analysis.
Results: Nano-implants retrieved after 90 days post-operatively depicted well-adhered, hexagonally arranged, and densely packed nanotubes with average diameter of 120±10 nm. The nanotubular architecture was generally well-preserved. Compared with the control bone volume percentage data (without GPC3), GPC3-loaded TNT/Ti without chitosan coating displayed a significantly lower volume percent in cranial CSDs (P<0.001). Histological assessment showed relatively less bone regeneration (healing) in GPC3-loaded CSDs than control CSDs.
Conclusion: The finding of inhibition of cranial bone regeneration by GPC3-loaded TNT/Ti in vivo is an important advance in the novel field of minimally-invasive craniosynostosis therapy and holds the prospect of altering the whole paradigm of treatment for affected children. Future animal studies on a larger sample are indicated to refine the dosage and duration of drug delivery across different ages and both sexes with the view to undertake human clinical trials.
Keywords: craniosynostosis; glypican; murine; protein delivery; titania nanotube.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures




References
-
- Gosain AK, McCarthy JG, Wisoff JH. Morbidity associated with increased intracranial pressure in Apert and Pfeiffer syndromes: the need for long-term evaluation. Plast Reconstr Surg. 1996;97(2):292–301. - PubMed
-
- Gonsalez S, Hayward R, Jones B, Lane R. Upper airway obstruction and raised intracranial pressure in children with craniosynostosis. Eur Respir J. 1997;10(2):367–375. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

