Capping gold nanoparticles with albumin to improve their biomedical properties
- PMID: 31496693
- PMCID: PMC6691944
- DOI: 10.2147/IJN.S210992
Capping gold nanoparticles with albumin to improve their biomedical properties
Abstract
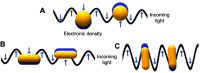
Nanotechnology is an emerging field which has created great opportunities either through the creation of new materials or by improving the properties of existing ones. Nanoscale materials with a wide range of applications in areas ranging from engineering to biomedicine have been produced. Gold nanoparticles (AuNPs) have emerged as a therapeutic agent, and are useful for imaging, drug delivery, and photodynamic and photothermal therapy. AuNPs have the advantage of ease of functionalization with therapeutic agents through covalent and ionic binding. Combining AuNPs and other materials can result in nanoplatforms, which can be useful for biomedical applications. Biomaterials such as biomolecules, polymers and proteins can improve the therapeutic properties of nanoparticles, such as their biocompatibility, biodistribution, stability and half-life. Serum albumin is a versatile, non-toxic, stable, and biodegradable protein, in which structural domains and functional groups allow the binding and capping of inorganic nanoparticles. AuNPs coated with albumin have improved properties such as greater compatibility, bioavailability, longer circulation times, lower toxicity, and selective bioaccumulation. In the current article, we review the features of albumin, as well as its interaction with AuNPs, focusing on its biomedical applications.
Keywords: albumin; drug delivery; photothermal therapy; theranostics.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures











Similar articles
-
Advances in Gold Nanoparticles: Synthesis, Functionalization Strategies, and Theranostic Applications in Cancer.Crit Rev Ther Drug Carrier Syst. 2024;41(6):1-56. doi: 10.1615/CritRevTherDrugCarrierSyst.2024046712. Crit Rev Ther Drug Carrier Syst. 2024. PMID: 38804553 Review.
-
Surface-bioengineered Gold Nanoparticles for Biomedical Applications.Curr Med Chem. 2018;25(16):1920-1944. doi: 10.2174/0929867325666180117111404. Curr Med Chem. 2018. PMID: 29345568 Review.
-
Synthesis, Chemical-Physical Characterization, and Biomedical Applications of Functional Gold Nanoparticles: A Review.Molecules. 2021 Sep 26;26(19):5823. doi: 10.3390/molecules26195823. Molecules. 2021. PMID: 34641367 Free PMC article. Review.
-
Gold nanoparticles enlighten the future of cancer theranostics.Int J Nanomedicine. 2017 Aug 22;12:6131-6152. doi: 10.2147/IJN.S140772. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28883725 Free PMC article. Review.
-
Biomedical applications of gold nanoparticles.Curr Top Med Chem. 2015;15(16):1605-13. doi: 10.2174/1568026615666150414144750. Curr Top Med Chem. 2015. PMID: 25877087 Review.
Cited by
-
Albumin Nanoparticle-Based Drug Delivery Systems.Int J Nanomedicine. 2024 Jul 10;19:6945-6980. doi: 10.2147/IJN.S467876. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39005962 Free PMC article. Review.
-
Smart Shockwave Responsive Titania-Based Nanoparticles for Cancer Treatment.Pharmaceutics. 2021 Sep 8;13(9):1423. doi: 10.3390/pharmaceutics13091423. Pharmaceutics. 2021. PMID: 34575499 Free PMC article.
-
Synthesis and in vitro assessment of gold nanoparticles conjugated with extracts, sterols and pure compounds derived from marine sponges from the Indian and Pacific Oceans.RSC Adv. 2024 Nov 11;14(48):36115-36131. doi: 10.1039/d4ra04068f. eCollection 2024 Nov 4. RSC Adv. 2024. PMID: 39529734 Free PMC article.
-
Ultra-Small Lysozyme-Protected Gold Nanoclusters as Nanomedicines Inducing Osteogenic Differentiation.Int J Nanomedicine. 2020 Jun 30;15:4705-4716. doi: 10.2147/IJN.S241163. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32636626 Free PMC article.
-
Therapeutic Nanodiamonds Containing Icariin Ameliorate the Progression of Osteoarthritis in Rats.Int J Mol Sci. 2023 Nov 5;24(21):15977. doi: 10.3390/ijms242115977. Int J Mol Sci. 2023. PMID: 37958960 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

