Antitumor effect of kurarinone and underlying mechanism in small cell lung carcinoma cells
- PMID: 31496721
- PMCID: PMC6689141
- DOI: 10.2147/OTT.S214964
Antitumor effect of kurarinone and underlying mechanism in small cell lung carcinoma cells
Abstract
Background: Kurarinone, a prenylated flavonone isolated from the roots of Sophora flavescens, is known to be cytotoxic against many human cancer cells but not human small cell lung carcinoma (SCLC) yet. Also, the exact molecular mechanism of kurarinone for induction cytotoxicity remains unknown.
Material and methods: We investigated the effects of kurarinone on cell proliferation, apoptosis, and migration in H1688 SCLC cells. Cell viability was determined by the MTT assay. Apoptotic indices such as cell cycle, mitochondrial membrane potential, cytochrome c release, caspase activity, and death receptors were evaluated by flow cytometry. Transwell migration and invasion assays were also included.
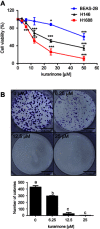
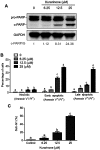
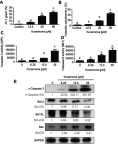
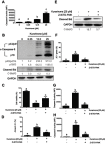
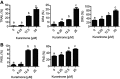
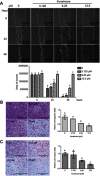
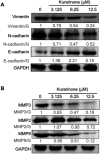
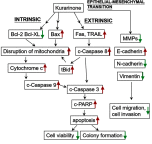
Results: Our results indicated that kurarinone significantly decreased H1688 cell viability and induced the accumulation of sub-G1 fractions by activating caspase-3, -9, and PARP cleavage accompanied by the elevated release of cytochrome c and mitochondrial dysfunction in H1688 cells. Additionally, kurarinone promoted Fas and TRAIL receptor-1 and -2 expression via the caspase-8/Bid pathway, suggesting that kurarinone triggered apoptosis via the mitochondria-mediated and receptor-mediated apoptotic pathways. We also observed that kurarinone repressed migration and invasion capabilities of SCLC cells by suppressing the expression of epithelial-mesenchymal transition-related proteins and matrix metalloproteinases.
Conclusion: Our findings provided evidence that kurarinone can induce apoptosis in SCLC cells via multiple mechanisms and delayed the cell migration and invasion of SCLC cells.
Keywords: apoptosis; caspase; invasiveness; kurarinone; migration; small cell lung carcinoma.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Five-Decade Update on Chemopreventive and Other Pharmacological Potential of Kurarinone: a Natural Flavanone.Front Pharmacol. 2021 Sep 27;12:737137. doi: 10.3389/fphar.2021.737137. eCollection 2021. Front Pharmacol. 2021. PMID: 34646138 Free PMC article. Review.
-
Kurarinone promotes TRAIL-induced apoptosis by inhibiting NF-κB-dependent cFLIP expression in HeLa cells.Exp Mol Med. 2012 Nov 30;44(11):653-64. doi: 10.3858/emm.2012.44.11.074. Exp Mol Med. 2012. PMID: 22932446 Free PMC article.
-
Inhibitory Effect of Kurarinone on Growth of Human Non-small Cell Lung Cancer: An Experimental Study Both in Vitro and in Vivo Studies.Front Pharmacol. 2018 Mar 23;9:252. doi: 10.3389/fphar.2018.00252. eCollection 2018. Front Pharmacol. 2018. PMID: 29628889 Free PMC article.
-
Evodiamine induces G2/M arrest and apoptosis via mitochondrial and endoplasmic reticulum pathways in H446 and H1688 human small-cell lung cancer cells.PLoS One. 2014 Dec 15;9(12):e115204. doi: 10.1371/journal.pone.0115204. eCollection 2014. PLoS One. 2014. PMID: 25506932 Free PMC article.
-
Kurarinone Synergizes TRAIL-Induced Apoptosis in Gastric Cancer Cells.Cell Biochem Biophys. 2015 May;72(1):241-9. doi: 10.1007/s12013-014-0444-0. Cell Biochem Biophys. 2015. PMID: 25524636
Cited by
-
Plant-derived glucose transport inhibitors with potential antitumor activity.Phytother Res. 2020 May;34(5):1027-1040. doi: 10.1002/ptr.6587. Epub 2019 Dec 10. Phytother Res. 2020. PMID: 31823431 Free PMC article. Review.
-
Role of Plant-Derived Natural Compounds in Experimental Autoimmune Encephalomyelitis: A Review of the Treatment Potential and Development Strategy.Front Pharmacol. 2021 Jun 28;12:639651. doi: 10.3389/fphar.2021.639651. eCollection 2021. Front Pharmacol. 2021. PMID: 34262447 Free PMC article. Review.
-
The Flavonoid Kurarinone Regulates Macrophage Functions via Aryl Hydrocarbon Receptor and Alleviates Intestinal Inflammation in Irritable Bowel Syndrome.J Inflamm Res. 2021 Sep 3;14:4347-4359. doi: 10.2147/JIR.S329091. eCollection 2021. J Inflamm Res. 2021. PMID: 34539182 Free PMC article.
-
Targeting Cell Signaling Pathways in Lung Cancer by Bioactive Phytocompounds.Cancers (Basel). 2023 Aug 5;15(15):3980. doi: 10.3390/cancers15153980. Cancers (Basel). 2023. PMID: 37568796 Free PMC article. Review.
-
Five-Decade Update on Chemopreventive and Other Pharmacological Potential of Kurarinone: a Natural Flavanone.Front Pharmacol. 2021 Sep 27;12:737137. doi: 10.3389/fphar.2021.737137. eCollection 2021. Front Pharmacol. 2021. PMID: 34646138 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

