OSM-induced CD44 contributes to breast cancer metastatic potential through cell detachment but not epithelial-mesenchymal transition
- PMID: 31496817
- PMCID: PMC6700398
- DOI: 10.2147/CMAR.S208721
OSM-induced CD44 contributes to breast cancer metastatic potential through cell detachment but not epithelial-mesenchymal transition
Abstract
Background: Hormone receptor status in human breast cancer cells is a strong indicator of the aggressiveness of a tumor. Triple negative breast cancers (TNBC) are aggressive, difficult to treat, and contribute to high incidences of metastasis by possessing characteristics such as increased tumor cell migration and a large presence of the transmembrane protein, cluster of differentiation 44 (CD44) on the cell membrane. Estrogen receptor-positive (ER+) cells are less aggressive and do not migrate until undergoing an epithelial-mesenchymal transition (EMT).
Methods: The relationship between EMT and CD44 during metastatic events is assessed by observing changes in EMT markers, tumor cell detachment, and migration following cytokine treatment on both parental and CD44 knockdown human breast tumor cells.
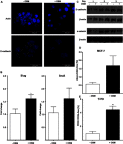
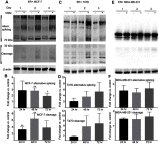
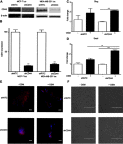
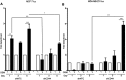
Results: ER+ T47D and MCF-7 human breast cancer cells treated with OSM demonstrate increased CD44 expression and CD44 cleavage. Conversely, ER- MDA-MB-231 human breast cancer cells do not show a change in CD44 expression nor undergo EMT in the presence of OSM. In ER+ cells, knockdown expression of CD44 by shRNA did not prevent EMT but did change metastatic processes such as cellular detachment and migration. OSM-induced migration was decreased in both ER+ and ER- cells with shCD44 cells compared to control cells, while the promotion of tumor cell detachment by OSM was decreased in ER+ MCF7-shCD44 cells, as compared to control cells. Interestingly, OSM-induced detachment in ER- MDA-MB-231-shCD44 cells that normally don't detach at significant rates.
Conclusion: OSM promotes both EMT and tumor cell detachment in ER+ breast cancer cells. Yet, CD44 knockdown did not affect OSM-induced EMT in these cells, while independently decreasing OSM-induced cell detachment. These results suggest that regulation of CD44 by OSM is important for at least part of the metastatic cascade in ER+ breast cancer.
Keywords: Oncostatin M; breast tumor metastasis; cluster of differentiation 44; epithelial to mesenchymal transition.
Conflict of interest statement
The authors declare that they have no competing interests in this work.
Figures


Similar articles
-
Oncostatin-M promotes phenotypic changes associated with mesenchymal and stem cell-like differentiation in breast cancer.Oncogene. 2014 Mar 20;33(12):1485-94. doi: 10.1038/onc.2013.105. Epub 2013 Apr 15. Oncogene. 2014. PMID: 23584474
-
The opposing effects of interferon-beta and oncostatin-M as regulators of cancer stem cell plasticity in triple-negative breast cancer.Breast Cancer Res. 2019 Apr 29;21(1):54. doi: 10.1186/s13058-019-1136-x. Breast Cancer Res. 2019. PMID: 31036052 Free PMC article.
-
Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states.Br J Cancer. 2014 Mar 18;110(6):1497-505. doi: 10.1038/bjc.2014.80. Epub 2014 Feb 25. Br J Cancer. 2014. PMID: 24569463 Free PMC article.
-
Nuclear beta-catenin and CD44 upregulation characterize invasive cell populations in non-aggressive MCF-7 breast cancer cells.BMC Cancer. 2010 Aug 10;10:414. doi: 10.1186/1471-2407-10-414. BMC Cancer. 2010. PMID: 20696077 Free PMC article.
-
Epithelial-to-mesenchymal transition and invadopodia markers in breast cancer: Lumican a key regulator.Semin Cancer Biol. 2020 May;62:125-133. doi: 10.1016/j.semcancer.2019.08.003. Epub 2019 Aug 8. Semin Cancer Biol. 2020. PMID: 31401293 Review.
Cited by
-
Expression of pyroptosis-related genes are correlated with immune microenvironment and predict prognosis in ESCA.J Cancer Res Clin Oncol. 2023 Sep;149(12):10701-10713. doi: 10.1007/s00432-023-04958-x. Epub 2023 Jun 12. J Cancer Res Clin Oncol. 2023. PMID: 37302999 Free PMC article.
-
The Role of the IL-6 Cytokine Family in Epithelial-Mesenchymal Plasticity in Cancer Progression.Int J Mol Sci. 2021 Aug 3;22(15):8334. doi: 10.3390/ijms22158334. Int J Mol Sci. 2021. PMID: 34361105 Free PMC article. Review.
-
The clinical relevance of OSM in inflammatory diseases: a comprehensive review.Front Immunol. 2023 Sep 29;14:1239732. doi: 10.3389/fimmu.2023.1239732. eCollection 2023. Front Immunol. 2023. PMID: 37841259 Free PMC article. Review.
-
TCF-3-mediated transcription of lncRNA HNF1A-AS1 targeting oncostatin M expression inhibits epithelial-mesenchymal transition via TGFβ signaling in gastroenteropancreatic neuroendocrine neoplasms.Aging (Albany NY). 2021 May 25;13(10):14065-14077. doi: 10.18632/aging.203024. Epub 2021 May 25. Aging (Albany NY). 2021. PMID: 34037532 Free PMC article.
-
Phyto-Immunotherapy, a Complementary Therapeutic Option to Decrease Metastasis and Attack Breast Cancer Stem Cells.Front Oncol. 2020 Aug 7;10:1334. doi: 10.3389/fonc.2020.01334. eCollection 2020. Front Oncol. 2020. PMID: 32850424 Free PMC article. Review.
References
-
- Scully OJ, Bay B-H, Yip G, Yu Y. Breast cancer metastasis. Cancer Genomics Proteomics [Internet] 2012;9:311–320. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22990110. Accessed July 5, 2019. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

