Antitumor profiles and cardiac electrophysiological effects of aurora kinase inhibitor ZM447439
- PMID: 31496876
- PMCID: PMC6717794
- DOI: 10.4196/kjpp.2019.23.5.393
Antitumor profiles and cardiac electrophysiological effects of aurora kinase inhibitor ZM447439
Abstract
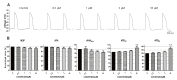
Aurora kinases inhibitors, including ZM447439 (ZM), which suppress cell division, have attracted a great deal of attention as potential novel anti-cancer drugs. Several recent studies have confirmed the anti-cancer effects of ZM in various cancer cell lines. However, there have been no studies regarding the cardiac safety of this agent. We performed several cytotoxicity, invasion and migration assays to examine the anti-cancer effects of ZM. To evaluate the potential effects of ZM on cardiac repolarisation, whole-cell patch-clamp experiments were performed with human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) and cells with heterogeneous cardiac ion channel expression. We also conducted a contractility assay with rat ventricular myocytes to determine the effects of ZM on myocardial contraction and/or relaxation. In tests to determine in vitro efficacy, ZM inhibited the proliferation of A549, H1299 (lung cancer), MCF-7 (breast cancer) and HepG2 (hepatoma) cell lines with IC50 in the submicromolar range, and attenuated the invasive and metastatic capacity of A549 cells. In cardiac toxicity testing, ZM did not significantly affect I Na, I Ks or I K1, but decreased I hERG in a dose-dependent manner (IC50: 6.53 µM). In action potential (AP) assay using hiPSC-CMs, ZM did not induce any changes in AP parameters up to 3 µM, but it at 10 µM induced prolongation of AP duration. In summary, ZM showed potent broad-spectrum anti-tumor activity, but relatively low levels of cardiac side effects compared to the effective doses to tumor. Therefore, ZM has a potential to be a candidate as an anti-cancer with low cardiac toxicity.
Keywords: Anticancer agents; Aurora kinases inhibitor; Cardiotoxicity; Efficacy testing; ZM447439.
Conflict of interest statement
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
Figures





Similar articles
-
Evaluation of nefazodone-induced cardiotoxicity in human induced pluripotent stem cell-derived cardiomyocytes.Toxicol Appl Pharmacol. 2016 Apr 1;296:42-53. doi: 10.1016/j.taap.2016.01.015. Epub 2016 Jan 25. Toxicol Appl Pharmacol. 2016. PMID: 26821276
-
Aurora kinase inhibitor ZM447439 induces apoptosis via mitochondrial pathways.Biochem Pharmacol. 2010 Jan 15;79(2):122-9. doi: 10.1016/j.bcp.2009.08.011. Epub 2009 Aug 15. Biochem Pharmacol. 2010. PMID: 19686703
-
Validating Aurora B as an anti-cancer drug target.J Cell Sci. 2006 Sep 1;119(Pt 17):3664-75. doi: 10.1242/jcs.03145. Epub 2006 Aug 15. J Cell Sci. 2006. PMID: 16912073
-
Moving beyond the comprehensive in vitro proarrhythmia assay: Use of human-induced pluripotent stem cell-derived cardiomyocytes to assess contractile effects associated with drug-induced structural cardiotoxicity.J Appl Toxicol. 2018 Sep;38(9):1166-1176. doi: 10.1002/jat.3611. Epub 2018 Feb 27. J Appl Toxicol. 2018. PMID: 29484688 Review.
-
Cardiotoxicity Assessment of Drugs Using Human iPS Cell-Derived Cardiomyocytes: Toward Proarrhythmic Risk and Cardio-Oncology.Curr Pharm Biotechnol. 2020;21(9):765-772. doi: 10.2174/1389201020666190628143345. Curr Pharm Biotechnol. 2020. PMID: 31264543 Review.
References
-
- Cirak Y, Furuncuoglu Y, Yapicier O, Aksu A, Cubukcu E. Aurora A overexpression in breast cancer patients induces taxane resistance and results in worse prognosis. J BUON. 2015;20:1414–1419. - PubMed
-
- Zekri A, Lesan V, Ghaffari SH, Tabrizi MH, Modarressi MH. Gene amplification and overexpression of Aurora-C in breast and prostate cancer cell lines. Oncol Res. 2012;20:241–250. - PubMed
-
- Yang H, Ou CC, Feldman RI, Nicosia SV, Kruk PA, Cheng JQ. Aurora-A kinase regulates telomerase activity through c-Myc in human ovarian and breast epithelial cells. Cancer Res. 2004;64:463–467. - PubMed

