Nociceptive Pathway in the Cockroach Periplaneta americana
- PMID: 31496959
- PMCID: PMC6712093
- DOI: 10.3389/fphys.2019.01100
Nociceptive Pathway in the Cockroach Periplaneta americana
Abstract
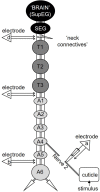
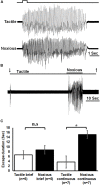
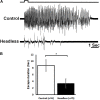
Detecting and avoiding environmental threats such as those with a potential for injury is of crucial importance for an animal's survival. In this work, we examine the nociceptive pathway in an insect, the cockroach Periplaneta americana, from detection of noxious stimuli to nocifensive behavior. We show that noxious stimuli applied to the cuticle of cockroaches evoke responses in sensory axons that are distinct from tactile sensory axons in the sensory afferent nerve. We also reveal differences in the evoked response of post-synaptic projection interneurons in the nerve cord to tactile versus noxious stimuli. Noxious stimuli are encoded in the cockroach nerve cord by fibers of diameter different from that of tactile and wind sensitive fibers with a slower conduction velocity of 2-3 m/s. Furthermore, recording from the neck-connectives show that the nociceptive information reaches the head ganglia. Removing the head ganglia results in a drastic decrease in the nocifensive response indicating that the head ganglia and the nerve cord are both involved in processing noxious stimuli.
Keywords: extracellular recording; insect; interneurons; nociception; nociceptive receptor; nocifensive behavior.
Figures




References
-
- Diwaker A. B. (1973). Distribution and Orientation of Campaniform Sensilla on the Abdomen of the Cockroaches Periplaneta Americana and Blaberus Cranifer. Emporia, KS: Emporia State University.
-
- Dyakonova V. (2001). Role of opioid peptides in behavior of invertebrates. J. Evol. Biochem. Physiol. 37 335–347.
LinkOut - more resources
Full Text Sources

