Time-Restricted Feeding Improves Glucose Tolerance in Rats, but Only When in Line With the Circadian Timing System
- PMID: 31496992
- PMCID: PMC6712481
- DOI: 10.3389/fendo.2019.00554
Time-Restricted Feeding Improves Glucose Tolerance in Rats, but Only When in Line With the Circadian Timing System
Abstract
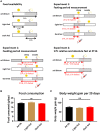
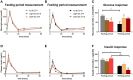
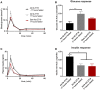
Epidemiological studies indicate that shift-workers have an increased risk of type 2 diabetes mellitus (T2DM). Glucose tolerance and insulin sensitivity both are dependent on the circadian timing system (i.e., the time-of-day) and fasting duration, in rodents as well as humans. Therefore, question is whether manipulation of the circadian timing system, for example by changing the timing of feeding and fasting, is a potential preventive treatment for T2DM. Time-restricted feeding (TRF) is well-known to have profound effects on various metabolic measures, including glucose metabolism. However, experiments that directly measure the effects of TRF on glucose tolerance and/or insulin sensitivity at different time points throughout the 24 h cycle are lacking. Here we show, in rats, that TRF in line with the circadian timing system (i.e., feeding during the active phase) improves glucose tolerance during intravenous glucose tolerance tests (ivGTT) in the active phase, as lower insulin levels were observed with similar levels of glucose clearance. However, this was not the case during the inactive phase in which more insulin was released but only a slightly faster glucose clearance was observed. Contrasting, TRF out of sync with the circadian timing system (i.e., feeding during the inactive phase) worsened glucose tolerance, although only marginally, likely because of adaptation to the 4 week TRF regimen. Our results show that TRF can improve glucose metabolism, but strict adherence to the time-restricted feeding period is necessary, as outside the regular eating hours glucose tolerance is worsened.
Keywords: Type 2 diabetes mellitus (T2DM); feeding behavior; insulin sensitivity; intravenous glucose tolerance test (ivGTT); metabolism; shift-work.
Figures
References
-
- Emerging Risk Factors Collaboration. Sarwar N, Gao P, Seshasai SRK, Gobin R, Kaptoge S, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. (2010) 375:2215–22. 10.1016/S0140-6736(10)60484-9 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

