HIV Infection Is Associated With Downregulation of BTLA Expression on Mycobacterium tuberculosis-Specific CD4 T Cells in Active Tuberculosis Disease
- PMID: 31497018
- PMCID: PMC6712065
- DOI: 10.3389/fimmu.2019.01983
HIV Infection Is Associated With Downregulation of BTLA Expression on Mycobacterium tuberculosis-Specific CD4 T Cells in Active Tuberculosis Disease
Abstract
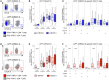
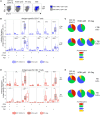
Nearly a quarter of the global population is infected with Mycobacterium tuberculosis (Mtb), with 10 million people developing active tuberculosis (TB) annually. Co-infection with human immunodeficiency virus (HIV) has long been recognized as a significant risk factor for progression to TB disease, yet the mechanisms whereby HIV impairs T cell-mediated control of Mtb infection remain poorly defined. We hypothesized that HIV infection may promote upregulation of inhibitory receptors on Mtb-specific CD4 T cells, a mechanism that has been associated with antigen-specific T cell dysfunction in chronic infections. Using cohorts of HIV-infected and HIV-uninfected individuals with latent Mtb infection (LTBI) and with active TB disease, we stimulated peripheral blood mononuclear cells (PBMC) for 6 hours with Mtb peptide pools and evaluated co-expression profiles of the inhibitory receptors BTLA, CTLA-4, and PD-1 on IFN-γ+/TNF-α+ Mtb-specific CD4 T cells. Mtb-specific CD4 T cells in all participant groups expressed predominately either one or no inhibitory receptors, unlike cytomegalovirus- and HIV-specific CD4 T cells circulating in the same individuals, which were predominately CTLA-4+PD-1+. There were no significant differences in inhibitory receptor expression profiles of Mtb-specific CD4 T cells between HIV-uninfected and HIV-infected individuals with LTBI. Surprisingly, BTLA expression, both alone and in combination with CTLA-4 and PD-1, was markedly downregulated on Mtb-specific CD4 T cells in HIV-infected individuals with active TB. Together, these data provide novel evidence that the majority of Mtb-specific CD4 T cells do not co-express multiple inhibitory receptors, regardless of HIV infection status; moreover, they highlight a previously unrecognized role of BTLA expression on Mtb-specific CD4 T cells that could be further explored as a potential biomarker of Mtb infection status, particularly in people living with HIV, the population at greatest risk for development of active TB disease.
Keywords: BTLA; CD4 T cell; CTLA-4; HIV; LTBI; Mycobacterium tuberculosis; PD-1; active TB disease.
Figures



References
-
- WHO Publication. Global Tuberculosis Report 2018. (2018). Available online at: http://www.who.int/tb/publications/global_report/en/ (accessed February 5, 2019).
-
- Day CL, Abrahams DA, Harris LD, van Rooyen M, Stone L, de Kock M, et al. . HIV-1 infection is associated with depletion and functional impairment of Mycobacterium tuberculosis-specific CD4 T cells in individuals with latent tuberculosis infection. J Immunol. (2017) 199:2069–80. 10.4049/jimmunol.1700558 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

