Conventional and Chemically Programmed Asymmetric Bispecific Antibodies Targeting Folate Receptor 1
- PMID: 31497024
- PMCID: PMC6712926
- DOI: 10.3389/fimmu.2019.01994
Conventional and Chemically Programmed Asymmetric Bispecific Antibodies Targeting Folate Receptor 1
Abstract
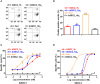
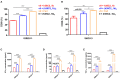
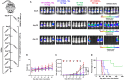
T-cell engaging bispecific antibodies (biAbs) can mediate potent and specific tumor cell eradication in liquid cancers. Substantial effort has been invested in expanding this concept to solid cancers. To explore their utility in the treatment of ovarian cancer, we built a set of asymmetric biAbs in IgG1-like format that bind CD3 on T cells with a conventional scFv arm and folate receptor 1 (FOLR1) on ovarian cancer cells with a conventional or a chemically programmed Fab arm. For avidity engineering, we also built an asymmetric biAb format with a tandem Fab arm. We show that both conventional and chemically programmed CD3 × FOLR1 biAbs exert specific in vitro and in vivo cytotoxicity toward FOLR1-expressing ovarian cancer cells by recruiting and activating T cells. While the conventional T-cell engaging biAb was curative in an aggressive mouse model of human ovarian cancer, the potency of the chemically programmed biAb was significantly boosted by avidity engineering. Both conventional and chemically programmed CD3 × FOLR1 biAbs warrant further investigation for ovarian cancer immunotherapy.
Keywords: CD3; FOLR1; bispecific antibodies; catalytic antibodies; folate; ovarian cancer.
Figures
Similar articles
-
Tumor-restricted activation of Vγ9Vδ2 T cells via bispecific Evobodies: a novel strategy for safe and potent immunotherapy in ovarian cancer.Front Immunol. 2025 Jul 18;16:1628501. doi: 10.3389/fimmu.2025.1628501. eCollection 2025. Front Immunol. 2025. PMID: 40755782 Free PMC article.
-
Chemically Programmed Bispecific Antibodies in Diabody Format.J Biol Chem. 2016 Sep 9;291(37):19661-73. doi: 10.1074/jbc.M116.745588. Epub 2016 Jul 21. J Biol Chem. 2016. PMID: 27445334 Free PMC article.
-
Chemically programmed bispecific antibodies that recruit and activate T cells.J Biol Chem. 2012 Aug 17;287(34):28206-14. doi: 10.1074/jbc.M112.384594. Epub 2012 Jul 3. J Biol Chem. 2012. PMID: 22761439 Free PMC article.
-
Redirecting cytotoxic T cells with chemically programmed antibodies.Bioorg Med Chem. 2020 Dec 15;28(24):115834. doi: 10.1016/j.bmc.2020.115834. Epub 2020 Nov 2. Bioorg Med Chem. 2020. PMID: 33166926 Review.
-
Bispecific antibodies in cancer immunotherapy.Curr Opin Biotechnol. 2020 Oct;65:9-16. doi: 10.1016/j.copbio.2019.11.020. Epub 2019 Dec 13. Curr Opin Biotechnol. 2020. PMID: 31841859 Free PMC article. Review.
Cited by
-
Chemically Programmable and Switchable CAR-T Therapy.Angew Chem Int Ed Engl. 2020 Jul 13;59(29):12178-12185. doi: 10.1002/anie.202005432. Epub 2020 May 18. Angew Chem Int Ed Engl. 2020. PMID: 32329959 Free PMC article.
-
Site-Specific Lysine Arylation as an Alternative Bioconjugation Strategy for Chemically Programmed Antibodies and Antibody-Drug Conjugates.Bioconjug Chem. 2019 Nov 20;30(11):2889-2896. doi: 10.1021/acs.bioconjchem.9b00609. Epub 2019 Nov 1. Bioconjug Chem. 2019. PMID: 31675216 Free PMC article.
-
Overcoming Challenges for CD3-Bispecific Antibody Therapy in Solid Tumors.Cancers (Basel). 2021 Jan 14;13(2):287. doi: 10.3390/cancers13020287. Cancers (Basel). 2021. PMID: 33466732 Free PMC article. Review.
-
Tumor-restricted activation of Vγ9Vδ2 T cells via bispecific Evobodies: a novel strategy for safe and potent immunotherapy in ovarian cancer.Front Immunol. 2025 Jul 18;16:1628501. doi: 10.3389/fimmu.2025.1628501. eCollection 2025. Front Immunol. 2025. PMID: 40755782 Free PMC article.
References
-
- Jen EY, Xu Q, Schetter A, Przepiorka D, Shen YL, Roscoe D, et al. . FDA approval: blinatumomab for patients with B-cell precursor acute lymphoblastic leukemia in morphologic remission with minimal residual disease. Clin Cancer Res. Cancer Res. (2019) 25:473–7. 10.1158/1078-0432.CCR-18-2337 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

