Airway CD8+CD161++TCRvα7.2+ T Cell Depletion During Untreated HIV Infection Targets CD103 Expressing Cells
- PMID: 31497028
- PMCID: PMC6713019
- DOI: 10.3389/fimmu.2019.02003
Airway CD8+CD161++TCRvα7.2+ T Cell Depletion During Untreated HIV Infection Targets CD103 Expressing Cells
Abstract
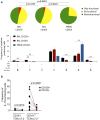
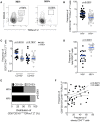
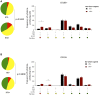
HIV-infected adults are at an increased risk to lower respiratory tract infections (LRTIs). CD8+CD161++TCRvα7.2+ T cells are an innate-like T cell subset that are thought to play an important role in early defense against pathogens in the respiratory tract. HIV infection leads to irreversible depletion of these cells in peripheral blood, however, its impact on this subset in the human airway is still unclear. Here, we show presence of CD103 expressing CD8+CD161++TCRvα7.2+ T cells in the airway that exhibited a distinct cytokine functional profile compared to their CD103- airway counterparts and those from peripheral blood. These CD103 expressing airway CD8+CD161++TCRvα7.2+ T cells were selectively depleted in untreated HIV-infected adults compared to healthy controls. Their frequency was positively correlated with frequency of airway CD4+ T cells. Furthermore, the frequency of airway CD8+CD161++TCRvα7.2+ T cells was also inversely correlated with HIV plasma viral load, while suppressive antiretroviral therapy (ART) resulted in restoration of airway CD8+CD161++TCRvα7.2+ T cells. Our findings show that CD103 expressing airway CD8+CD161++TCRvα7.2+ T cells are functionally distinct and are preferentially depleted during untreated asymptomatic HIV infection. Depletion of CD103 expressing airway CD8+CD161++TCRvα7.2+ T cells, at a major portal of pathogen entry, could partly contribute to the increased propensity for opportunistic LRTIs observed in untreated HIV-infected adults.
Keywords: CD103; CD8 T cell; HIV; adult; airway.
Figures



References
-
- Murray JF. Pulmonary complications of HIV-1 infection among adults living in Sub-Saharan Africa. Int J Tuberc Lung Dis. (2005) 9:826. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

