Short-term stent coverage of second-generation zotarolimus-eluting durable polymer stents: Onyx one-month optical coherence tomography study
- PMID: 31497046
- PMCID: PMC6727229
- DOI: 10.5114/aic.2019.86009
Short-term stent coverage of second-generation zotarolimus-eluting durable polymer stents: Onyx one-month optical coherence tomography study
Abstract
Introduction: To date the early strut coverage with the second-generation durable-polymer ONYX zotarolimus-eluting stent (O-ZES) is unknown.
Aim: Optical coherence tomography (OCT) assessed the strut coverage of O-ZES at thirty-day follow-up.
Material and methods: OCT was performed after implantation and at 1-month follow-up in 15 patients treated with O-ZES.
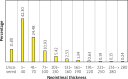
Results: Mean patient age was 67 ±7 years (73% males). The clinical presentation consisted of acute coronary syndromes (n = 13) and stable coronary disease (n = 2). Four (26%) patients had diabetes. OCT analysis was performed at baseline and 1-month follow-up in all stents. 378 cross-sections with 3582 struts were assessed at baseline and 3661 at follow-up. At follow-up, 88% struts were covered by tissue with a median thickness 37.91 μm (IQR: 22.32-64.15). Median in-stent area obstruction by neointima was 2.64% (IQR: 1.70-4.84). From the total stent covered area, 92.3% showed complete strut coverage. Homogeneous tissue was observed in 74% of cases. There were no differences in minimal lumen area (5.07 ±1.08 mm2 vs. 4.81 ±0.94 mm2, p = 0.125) or minimal stent area (4.95 ±1.22 mm2 vs. 4.92 ±0.99 mm2) at baseline and at follow-up. There were no differences in the rate of strut malapposition (4.3% vs. 5.7%, p = 0.417). For all stents, malapposition volume was 47.9 mm3 at baseline and 51.7 mm3 at follow-up, giving the late acquired stent malapposition volume of 3.8 mm3.
Conclusions: The second-generation durable polymer O-ZES showed favorable vessel healing at 30-day OCT follow-up.
Keywords: 1-month follow-up; optical coherence tomography; vessel healing; zotarolimus-eluting stent.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Evaluation in 3 months duration of neointimal coverage after zotarolimus-eluting stent implantation by optical coherence tomography: the ENDEAVOR OCT trial.JACC Cardiovasc Interv. 2009 Dec;2(12):1240-7. doi: 10.1016/j.jcin.2009.10.006. JACC Cardiovasc Interv. 2009. PMID: 20129551 Clinical Trial.
-
Strut coverage and vessel wall response to zotarolimus-eluting and bare-metal stents implanted in patients with ST-segment elevation myocardial infarction: the OCTAMI (Optical Coherence Tomography in Acute Myocardial Infarction) Study.JACC Cardiovasc Interv. 2010 Jun;3(6):680-7. doi: 10.1016/j.jcin.2010.04.005. JACC Cardiovasc Interv. 2010. PMID: 20630463 Clinical Trial.
-
Comparison of early strut coverage between zotarolimus- and everolimus-eluting stents using optical coherence tomography.Am J Cardiol. 2013 Jan 1;111(1):1-5. doi: 10.1016/j.amjcard.2012.08.037. Epub 2012 Oct 2. Am J Cardiol. 2013. PMID: 23040589 Clinical Trial.
-
Optical coherence tomography analysis of the stent strut and prediction of resolved strut malapposition at 3 months after 2nd-generation drug-eluting stent implantation.Heart Vessels. 2016 Aug;31(8):1247-56. doi: 10.1007/s00380-015-0737-2. Epub 2015 Sep 3. Heart Vessels. 2016. PMID: 26334709
-
Late stent strut apposition and coverage after drug-eluting stent implantation by optical coherence tomography in patients with acute myocardial infarction.Coron Artery Dis. 2025 May 27. doi: 10.1097/MCA.0000000000001536. Online ahead of print. Coron Artery Dis. 2025. PMID: 40421609
Cited by
-
Cardiovascular Stents: A Review of Past, Current, and Emerging Devices.Materials (Basel). 2021 May 12;14(10):2498. doi: 10.3390/ma14102498. Materials (Basel). 2021. PMID: 34065986 Free PMC article. Review.
-
Assessment of Effectiveness of the Algorithm for Automated Quantitative Analysis of Metallic Strut Tissue Short-Term Coverage with Intravascular Optical Coherence Tomography.J Clin Med. 2024 Jul 25;13(15):4336. doi: 10.3390/jcm13154336. J Clin Med. 2024. PMID: 39124606 Free PMC article.
-
Effects of exenatide on coronary stent's endothelialization in subjects with type 2 diabetes: a randomized controlled trial. The Rebuild study.Cardiovasc Diabetol. 2023 Dec 8;22(1):337. doi: 10.1186/s12933-023-02071-4. Cardiovasc Diabetol. 2023. PMID: 38066597 Free PMC article. Clinical Trial.
-
A prospective, multicentre first-in-man study of the polymer-free ultrathin-strut BIOrapid stent (BIOVITESSE).EuroIntervention. 2022 Jun 3;18(2):e132-e139. doi: 10.4244/EIJ-D-21-00537. EuroIntervention. 2022. PMID: 34794936 Free PMC article.
-
Importance of Short-Term Neointimal Coverage of Drug-Eluting Stents in the Duration of Dual Antiplatelet Therapy.J Clin Med. 2024 Mar 17;13(6):1730. doi: 10.3390/jcm13061730. J Clin Med. 2024. PMID: 38541955 Free PMC article. Review.
References
-
- Finn AV, Nakazawa G, Joner M, et al. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27:1500–10. - PubMed
-
- Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937–48. - PubMed
-
- Palmerini T, Benedetto U, Bacchi-Reggiani L, et al. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet. 2015;385:2371–82. - PubMed
-
- Zhang Y, Tian N, Dong S, et al. Impact of biodegradable versus durable polymer drug-eluting stents on clinical outcomes in patients with coronary artery disease: a meta-analysis of 15 randomized trials. Chin Med J (Engl) 2014;127:2159–66. - PubMed
-
- Urban P, Meredith IT, Abizaid A, et al. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med. 2015;373:2038–47. - PubMed
LinkOut - more resources
Full Text Sources
