Clinical Trial Publication Trends Within Neurology
- PMID: 31497319
- PMCID: PMC6708287
- DOI: 10.1515/tnsci-2019-0037
Clinical Trial Publication Trends Within Neurology
Abstract
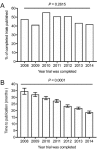
Timely dissemination of results from clinical studies is crucial for the advancement of knowledge and clinical decision making. A large body of research has shown that up to half of clinical trials do not publish their findings. In this study, we sought to determine whether clinical trial publication rates within neurology have increased over time. Focusing on neurology clinical trials completed between 2008 to 2014, we found that while the overall percentage of published trials has not changed (remaining at approximately 50%), time to publication has significantly decreased. Our findings suggest that clinical trials within neurology are being published in a more timely manner.
Keywords: clinical research; clinical trials; clinicaltrials.gov; neurology; neuroscience.
Figures
References
-
- The Food and Drug Administration Amendments Act (FDAAA) Public Law: 110-85. 2007. T.F.a.D. Administration, Editor.
Grants and funding
LinkOut - more resources
Full Text Sources
