HSF2 regulates aerobic glycolysis by suppression of FBP1 in hepatocellular carcinoma
- PMID: 31497345
- PMCID: PMC6726997
HSF2 regulates aerobic glycolysis by suppression of FBP1 in hepatocellular carcinoma
Abstract
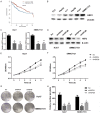
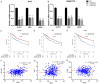
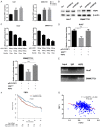
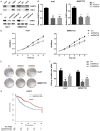
Heat shock factors (HSFs) are essential for all organisms to survive exposures to acute stress. Recent years have witnessed the progress in uncovering the importance of HSFs in cancer cell oncogenesis, progression and metastasis. However, their roles in hepatocellular carcinoma (HCC) proliferation and the underlying mechanism have seldom been discussed. The present study aims to uncover the two important HSFs members HSF1 and HSF2 in hepatocellular carcinoma (HCC). By using the Cancer Genome Atlas (TCGA) dataset analysis, we investigated the prognosis value of HSF1 and HSF2 in HCC and identified HSF2 as a prediction factor of overall survival of HCC. In vitro cell line studies demonstrated that silencing HSF2 expression could decrease the proliferation in HCC cells. In depth mechanism analysis demonstrated that HSF2 promoted cell proliferation via positive regulation of aerobic glycolysis, and HSF2 interacted with euchromatic histone lysine methyltransferase 2 (EHMT2) to epigenetically silence fructose-bisphosphatase 1 (FBP1), which is a tumor suppressor and negative regulator of aerobic glycolysis in HCC. HSF2 expression indicated unfavorable prognosis of HCC patients and it could regulate aerobic glycolysis by suppression of FBP1 to support uncontrolled proliferation of HCC cells.
Keywords: Hepatocellular carcinoma; aerobic glycolysis; euchromatic histone lysine methyltransferase 2; fructose-bisphosphatase 1; heat shock factor 2.
Conflict of interest statement
None.
Figures


Similar articles
-
FOXP2 suppresses the proliferation, invasion, and aerobic glycolysis of hepatocellular carcinoma cells by regulating the KDM5A/FBP1 axis.Environ Toxicol. 2024 Jan;39(1):341-356. doi: 10.1002/tox.23971. Epub 2023 Sep 15. Environ Toxicol. 2024. PMID: 37713600
-
Decreased Expression of Fructose-1,6-bisphosphatase Associates with Glucose Metabolism and Tumor Progression in Hepatocellular Carcinoma.Cancer Res. 2016 Jun 1;76(11):3265-76. doi: 10.1158/0008-5472.CAN-15-2601. Epub 2016 Apr 6. Cancer Res. 2016. PMID: 27197151
-
Identification, tissue distribution and characterization of two heat shock factors (HSFs) in goldfish (Carassius auratus).Fish Shellfish Immunol. 2015 Apr;43(2):375-86. doi: 10.1016/j.fsi.2015.01.004. Epub 2015 Jan 12. Fish Shellfish Immunol. 2015. PMID: 25592877
-
Heat shock factors at a crossroad between stress and development.Ann N Y Acad Sci. 2007 Oct;1113:15-27. doi: 10.1196/annals.1391.005. Epub 2007 May 4. Ann N Y Acad Sci. 2007. PMID: 17483205 Review.
-
Reprogramming of glucose metabolism in hepatocellular carcinoma: Progress and prospects.World J Gastroenterol. 2016 Dec 7;22(45):9933-9943. doi: 10.3748/wjg.v22.i45.9933. World J Gastroenterol. 2016. PMID: 28018100 Free PMC article. Review.
Cited by
-
The function and regulation of heat shock transcription factor in Cryptococcus.Front Cell Infect Microbiol. 2023 Apr 24;13:1195968. doi: 10.3389/fcimb.2023.1195968. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37168390 Free PMC article. Review.
-
Concanavalin A inhibits human liver cancer cell migration by regulating F-actin redistribution and assembly via MAPK signaling pathway.Oncol Lett. 2022 Sep 23;24(5):405. doi: 10.3892/ol.2022.13525. eCollection 2022 Nov. Oncol Lett. 2022. PMID: 36276493 Free PMC article.
-
HSF4 promotes tumor progression of colorectal cancer by transactivating c-MET.Mol Cell Biochem. 2023 May;478(5):1141-1150. doi: 10.1007/s11010-022-04582-2. Epub 2022 Oct 13. Mol Cell Biochem. 2023. PMID: 36229759
-
Integrated Bioinformatics Analysis Identifies Heat Shock Factor 2 as a Prognostic Biomarker Associated With Immune Cell Infiltration in Hepatocellular Carcinoma.Front Genet. 2021 Nov 30;12:668516. doi: 10.3389/fgene.2021.668516. eCollection 2021. Front Genet. 2021. PMID: 34917120 Free PMC article.
-
Characterization of neuroendocrine regulation- and metabolism-associated molecular features and prognostic indicators with aid to clinical chemotherapy and immunotherapy of patients with pancreatic cancer.Front Endocrinol (Lausanne). 2023 Jan 20;13:1078424. doi: 10.3389/fendo.2022.1078424. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36743929 Free PMC article.
References
-
- Lamarca A, Mendiola M, Barriuso J. Hepatocellular carcinoma: exploring the impact of ethnicity on molecular biology. Crit Rev Oncol Hematol. 2016;105:65–72. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
