Mycobacterium tuberculosis Primary Infection and Dissemination: A Critical Role for Alveolar Epithelial Cells
- PMID: 31497538
- PMCID: PMC6712944
- DOI: 10.3389/fcimb.2019.00299
Mycobacterium tuberculosis Primary Infection and Dissemination: A Critical Role for Alveolar Epithelial Cells
Abstract
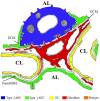
Globally, tuberculosis (TB) has reemerged as a major cause of morbidity and mortality, despite the use of the Mycobacterium bovis BCG vaccine and intensive attempts to improve upon BCG or develop new vaccines. Two lacunae in our understanding of the Mycobacterium tuberculosis (M. tb)-host pathogenesis have mitigated the vaccine efforts; the bacterial-host interaction that enables successful establishment of primary infection and the correlates of protection against TB. The vast majority of vaccine efforts are based on the premise that cell-mediated immunity (CMI) is the predominating mode of protection against TB. However, studies in animal models and in humans demonstrate that post-infection, a period of several weeks precedes the initiation of CMI during which the few inhaled bacteria replicate dramatically and disseminate systemically. The "Trojan Horse" mechanism, wherein M. tb is phagocytosed and transported across the alveolar barrier by infected alveolar macrophages has been long postulated as the sole, primary M. tb:host interaction. In the current review, we present evidence from our studies of transcriptional profiles of M. tb in sputum as it emerges from infectious patients where the bacteria are in a quiescent state, to its adaptations in alveolar epithelial cells where the bacteria transform to a highly replicative and invasive phenotype, to its maintenance of the invasive phenotype in whole blood to the downregulation of invasiveness upon infection of epithelial cells at an extrapulmonary site. Evidence for this alternative mode of infection and dissemination during primary infection is supported by in vivo, in vitro cell-based, and transcriptional studies from multiple investigators in recent years. The proposed alternative mechanism of primary infection and dissemination across the alveolar barrier parallels our understanding of infection and dissemination of other Gram-positive pathogens across their relevant mucosal barriers in that barrier-specific adhesins, toxins, and enzymes synergize to facilitate systemic establishment of infection prior to the emergence of CMI. Further exploration of this M. tb:non-phagocytic cell interaction can provide alternative approaches to vaccine design to prevent infection with M. tb and not only decrease clinical disease but also decrease the overwhelming reservoir of latent TB infection.
Keywords: adhesins; alveolar epithelial cells; infection; toxins; transcriptome; tuberculosis; vaccine.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

