From protein engineering to artificial enzymes - biological and biomimetic approaches towards sustainable hydrogen production
- PMID: 31497651
- PMCID: PMC6695573
- DOI: 10.1039/c7se00582b
From protein engineering to artificial enzymes - biological and biomimetic approaches towards sustainable hydrogen production
Abstract
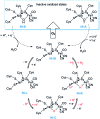
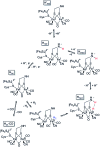
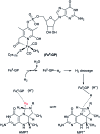
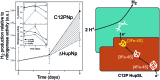
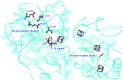
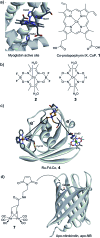
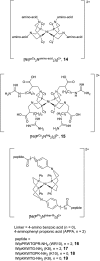
Hydrogen gas is used extensively in industry today and is often put forward as a suitable energy carrier due its high energy density. Currently, the main source of molecular hydrogen is fossil fuels via steam reforming. Consequently, novel production methods are required to improve the sustainability of hydrogen gas for industrial processes, as well as paving the way for its implementation as a future solar fuel. Nature has already developed an elaborate hydrogen economy, where the production and consumption of hydrogen gas is catalysed by hydrogenase enzymes. In this review we summarize efforts on engineering and optimizing these enzymes for biological hydrogen gas production, with an emphasis on their inorganic cofactors. Moreover, we will describe how our understanding of these enzymes has been applied for the preparation of bio-inspired/-mimetic systems for efficient and sustainable hydrogen production.
Figures






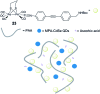




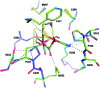
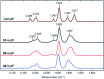

References
-
- Lauermann G., Häussinger P., Lohmüller R. and Watson A. M., in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2013, pp. 1–15, 10.1002/14356007.a13_297.pub3. - DOI
-
- Ogden J. M. Annu. Rev. Energy. 1999;24:227–279.
-
- Badwal S. P. S., Giddey S., Munnings C. Wiley Interdiscip. Rev.: Energy Environ. 2013;2:473–487.
-
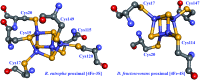
- Fontecilla-Camps J. C., Amara P., Cavazza C., Nicolet Y., Volbeda A. Nature. 2009;460:814–822. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
