Efficient Removal of Pb(II) and Cd(II) from Industrial Mine Water by a Hierarchical MoS2/SH-MWCNT Nanocomposite
- PMID: 31497710
- PMCID: PMC6714537
- DOI: 10.1021/acsomega.9b01603
Efficient Removal of Pb(II) and Cd(II) from Industrial Mine Water by a Hierarchical MoS2/SH-MWCNT Nanocomposite
Abstract
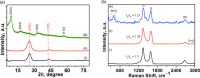
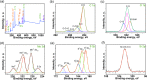
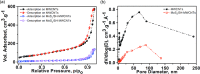
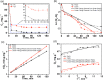
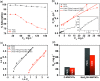
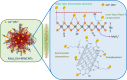
In this study, we investigate the adsorption capability of molybdenum sulfide (MoS2)/thiol-functionalized multiwalled carbon nanotube (SH-MWCNT) nanocomposite for rapid and efficient removal of heavy metals [Pb(II) and Cd(II)] from industrial mine water. The MoS2/SH-MWCNT nanocomposite was synthesized by acid treatment and sulfurization of MWCNTs followed by a facile hydrothermal reaction technique using sodium molybdate and diethyldithiocarbamate as MoS2 precursors. Morphological and chemical features of the nanocomposite material were studied using various characterization techniques. Furthermore, the effects of adsorbent (MoS2/SH-MWCNT nanocomposite) concentration, contact time, initial concentration of heavy-metal ions, and reaction temperature were examined to determine the efficiency of the adsorption process in batch adsorption experiments. Kinetics and isotherm studies showed that the adsorption process followed pseudo-second-order and Freundlich adsorption isotherm models, respectively. Thermodynamic parameters calculated using van't Hoff plots show the spontaneity and endothermic nature of adsorption. MoS2/SH-MWCNT nanocomposite demonstrates a high adsorption capacity for Pb(II) (90.0 mg g-1) and Cd(II) (66.6 mg g-1) following ion-exchange and electrostatic interactions. Metal-sulfur complex formation was identified as the key contributor for adsorption of heavy-metal ions followed by electrostatic interactions for multilayer adsorption. Transformation of adsorbent into PbMoO4-x S x and CdMoO4-x S x complex because of the adsorption process was confirmed by X-ray diffraction and scanning electron microscopy-energy-dispersive spectrometry. The spent adsorbent can further be used for photocatalytic and electrochemical applications; therefore, the generated secondary byproducts can also be employed for other purposes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Zhang Y.; Almodovar-Arbelo N. E.; Weidman J. L.; Corti D. S.; Boudouris B. W.; Phillip W. A. Fit-for-purpose block polymer membranes molecularly engineered for water treatment. npj Clean Water 2018, 1, 210.1038/s41545-018-0002-1. - DOI
-
- Sharma S.; Bhattacharya A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067. 10.1007/s13201-016-0455-7. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
