AntimiR-155 Cyclic Peptide-PNA Conjugate: Synthesis, Cellular Uptake, and Biological Activity
- PMID: 31497713
- PMCID: PMC6714607
- DOI: 10.1021/acsomega.9b01697
AntimiR-155 Cyclic Peptide-PNA Conjugate: Synthesis, Cellular Uptake, and Biological Activity
Abstract
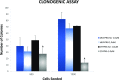
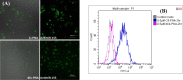
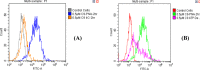
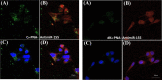
Efficient delivery of nucleic acids into cells still remains a great challenge. Peptide nucleic acids (PNAs) are DNA analogues with a neutral backbone and are synthesized by solid phase peptide chemistry. This allows a straightforward synthetic route to introduce a linear short peptide (a.k.a. cell-penetrating peptide) to the PNA molecule as a means of facilitating cellular uptake of PNAs. Herein, we have devised a synthetic route in which a cyclic peptide is prepared on a solid support and is extended with the PNA molecule, where all syntheses are accomplished on the solid phase. This allows the conjugation of the cyclic peptide to the PNA molecule with the need of only one purification step after the cyclic peptide-PNA conjugate (C9-PNA) is cleaved from the solid support. The PNA sequence chosen is an antimiR-155 molecule that is complementary to mature miR-155, a well-established oncogenic miRNA. By labeling C9-PNA with fluorescein isothiocyanate, we observe efficient cellular uptake into glioblastoma cells (U87MG) at a low concentration (0.5 μM), as corroborated by fluorescence-activated cell sorting (FACS) analysis and confocal microscopy. FACS analysis also suggests an uptake mechanism that is energy-dependent. Finally, the antimiR activity of C9-PNA was shown by analyzing miR155 levels by quantitative reverse transcription polymerase chain reaction and by observing a reduction in cell viability and proliferation in U87MG cells, as corroborated by XTT and colony formation assays. Given the added biological stability of cyclic versus linear peptides, this synthetic approach may be a useful and straightforward approach to synthesize cyclic peptide-PNA conjugates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

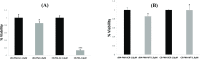
Similar articles
-
Intracellular uptake and inhibition of gene expression by PNAs and PNA-peptide conjugates.Biochemistry. 2004 Nov 16;43(45):14340-7. doi: 10.1021/bi048519l. Biochemistry. 2004. PMID: 15533038
-
Cell-dependent differential cellular uptake of PNA, peptides, and PNA-peptide conjugates.Antisense Nucleic Acid Drug Dev. 2002 Apr;12(2):51-63. doi: 10.1089/108729002760070795. Antisense Nucleic Acid Drug Dev. 2002. PMID: 12074365
-
Enhancement of intracellular concentration and biological activity of PNA after conjugation with a cell-penetrating synthetic model peptide.Eur J Biochem. 2004 Jul;271(14):3043-9. doi: 10.1111/j.1432-1033.2004.04236.x. Eur J Biochem. 2004. PMID: 15233801
-
Peptide-nucleic acids (PNAs): a tool for the development of gene expression modifiers.Curr Pharm Des. 2001 Nov;7(17):1839-62. doi: 10.2174/1381612013397087. Curr Pharm Des. 2001. PMID: 11562312 Review.
-
Biological activity and delivery of peptide nucleic acids (PNA)-DNA chimeras for transcription factor decoy (TFD) pharmacotherapy.Curr Med Chem. 2004 May;11(10):1253-63. doi: 10.2174/0929867043365242. Curr Med Chem. 2004. PMID: 15134518 Review.
Cited by
-
Efficient antisense inhibition reveals microRNA-155 to restrain a late-myeloid inflammatory programme in primary human phagocytes.RNA Biol. 2021 May;18(5):604-618. doi: 10.1080/15476286.2021.1885209. Epub 2021 Feb 23. RNA Biol. 2021. PMID: 33622174 Free PMC article.
-
Cytosolic delivery of peptidic STAT3 SH2 domain inhibitors.Bioorg Med Chem. 2020 Jun 15;28(12):115542. doi: 10.1016/j.bmc.2020.115542. Epub 2020 May 4. Bioorg Med Chem. 2020. PMID: 32503696 Free PMC article.
-
Discovery of a Cyclic Cell-Penetrating Peptide with Improved Endosomal Escape and Cytosolic Delivery Efficiency.Mol Pharm. 2022 May 2;19(5):1378-1388. doi: 10.1021/acs.molpharmaceut.1c00924. Epub 2022 Apr 11. Mol Pharm. 2022. PMID: 35405068 Free PMC article.
-
Enhancing the Cell Permeability of Stapled Peptides with a Cyclic Cell-Penetrating Peptide.J Med Chem. 2019 Nov 27;62(22):10098-10107. doi: 10.1021/acs.jmedchem.9b00456. Epub 2019 Nov 8. J Med Chem. 2019. PMID: 31657556 Free PMC article.
-
Engineering Cell-Permeable Proteins through Insertion of Cell-Penetrating Motifs into Surface Loops.ACS Chem Biol. 2020 Sep 18;15(9):2568-2576. doi: 10.1021/acschembio.0c00593. Epub 2020 Aug 20. ACS Chem Biol. 2020. PMID: 32786266 Free PMC article.
References
-
- Betts C.; Saleh A. F.; Arzumanov A. A.; Hammond S. M.; Godfrey C.; Coursindel T.; Gait M. J.; Wood M. J. A. Pip6-PMO, A New Generation of Peptide-oligonucleotide Conjugates With Improved Cardiac Exon Skipping Activity for DMD Treatment. Mol. Ther. Nucleic Acids 2012, 1, e3810.1038/mtna.2012.30. - DOI - PMC - PubMed
- Clayton N. P.; Nelson C. A.; Weeden T.; Taylor K. M.; Moreland R. J.; Scheule R. K.; Phillips L.; Leger A. J.; Cheng S. H.; Wentworth B. M. Antisense Oligonucleotide-mediated Suppression of Muscle Glycogen Synthase 1 Synthesis as an Approach for Substrate Reduction Therapy of Pompe Disease. Mol. Ther. Nucleic Acids 2014, 3, e20610.1038/mtna.2014.57. - DOI - PMC - PubMed
- Echigoya Y.; Nakamura A.; Nagata T.; Urasawa N.; Lim K. R. Q.; Trieu N.; Panesar D.; Kuraoka M.; Moulton H. M.; Saito T.; Aoki Y.; Iversen P.; Sazani P.; Kole R.; Maruyama R.; Partridge T.; Takeda S. i.; Yokota T. Effects of systemic multiexon skipping with peptide-conjugated morpholinos in the heart of a dog model of Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. U.S.A. 2017, 114, 4213–4218. 10.1073/pnas.1613203114. - DOI - PMC - PubMed
- Gait M. J.; Arzumanov A. A.; McClorey G.; Godfrey C.; Betts C.; Hammond S.; Wood M. J. A. Cell-Penetrating Peptide Conjugates of Steric Blocking Oligonucleotides as Therapeutics for Neuromuscular Diseases from a Historical Perspective to Current Prospects of Treatment. Nucleic Acid Ther. 2019, 29, 1–12. 10.1089/nat.2018.0747. - DOI - PMC - PubMed
- Goyenvalle A.; Babbs A.; Powell D.; Kole R.; Fletcher S.; Wilton S. D.; Davies K. E. Prevention of Dystrophic Pathology in Severely Affected Dystrophin/Utrophin-deficient Mice by Morpholino-oligomer-mediated Exon-skipping. Mol. Ther. 2010, 18, 198–205. 10.1038/mt.2009.248. - DOI - PMC - PubMed
- Greenberg D. E.; Marshall-Batty K. R.; Brinster L. R.; Zarember K. A.; Shaw P. A.; Mellbye B. L.; Iversen P. L.; Holland S. M.; Geller B. L. Antisense Phosphorodiamidate Morpholino Oligomers Targeted to an Essential Gene Inhibit Burkholderia cepacia Complex. J. Infect. Dis. 2010, 201, 1822–1830. 10.1086/652807. - DOI - PMC - PubMed
- Hammond S. M.; Hazell G.; Shabanpoor F.; Saleh A. F.; Bowerman M.; Sleigh J. N.; Meijboom K. E.; Zhou H.; Muntoni F.; Talbot K.; Gait M. J.; Wood M. J. A. Systemic peptide-mediated oligonucleotide therapy improves long-term survival in spinal muscular atrophy. Proc. Natl. Acad. Sci. U.S.A. 2016, 113, 10962–10967. 10.1073/pnas.1605731113. - DOI - PMC - PubMed
- Jearawiriyapaisarn N.; Moulton H. M.; Buckley B.; Roberts J.; Sazani P.; Fucharoen S.; Iversen P. L.; Kole R. Sustained dystrophin expression induced by peptide-conjugated morpholino oligomers in the muscles of mdx mice. Mol. Ther. 2008, 16, 1624–1629. 10.1038/mt.2008.120. - DOI - PMC - PubMed
- Jearawiriyapaisarn N.; Moulton H. M.; Sazani P.; Kole R.; Willis M. S. Long-term improvement in mdx cardiomyopathy after therapy with peptide-conjugated morpholino oligomers. Cardiovasc. Res. 2010, 85, 444–453. 10.1093/cvr/cvp335. - DOI - PMC - PubMed
- Lai S.-H.; Stein D. A.; Guerrero-Plata A.; Liao S.-L.; Ivanciuc T.; Hong C.; Iversen P. L.; Casola A.; Garofalo R. P. Inhibition of respiratory syncytial virus infections with morpholino oligomers in cell cultures and in mice. Mol. Ther. 2008, 16, 1120–1128. 10.1038/mt.2008.81. - DOI - PMC - PubMed
- Lebleu B.; Moulton H. M.; Abes R.; Ivanova G. D.; Abes S.; Stein D. A.; Iversen P. L.; Arzumanov A. A.; Gait M. J. Cell penetrating peptide conjugates of steric block oligonucleotides. Adv. Drug Delivery Rev. 2008, 60, 517–529. 10.1016/j.addr.2007.09.002. - DOI - PMC - PubMed
- Leger A. J.; Mosquea L. M.; Clayton N. P.; Wu I.-H.; Weeden T.; Nelson C. A.; Phillips L.; Roberts E.; Piepenhagen P. A.; Cheng S. H.; Wentworth B. M. Systemic Delivery of a Peptide-Linked Morpholino Oligonucleotide Neutralizes Mutant RNA Toxicity in a Mouse Model of Myotonic Dystrophy. Nucleic Acid Ther. 2013, 23, 109–117. 10.1089/nat.2012.0404. - DOI - PubMed
- Ma W.; Lin Y.; Xuan W.; Iversen P. L.; Smith L. J.; Benchimol S. Inhibition of p53 expression by peptide-conjugated phosphorodiamidate morpholino oligomers sensitizes human cancer cells to chemotherapeutic drugs. Oncogene 2012, 31, 1024–1033. 10.1038/onc.2011.300. - DOI - PubMed
- Morse M. A.; Hobeika A.; Serra D.; Aird K.; McKinney M.; Aldrich A.; Clay T.; Mourich D.; Lyerly H. K.; Iversen P. L.; Devi G. R. Depleting regulatory T cells with arginine-rich, cell-penetrating, peptide-conjugated morpholino oligomer targeting FOXP3 inhibits regulatory T-cell function. Cancer Gene Ther. 2012, 19, 30–37. 10.1038/cgt.2011.63. - DOI - PubMed
- Moulton H. M.; Moulton J. D. Morpholinos and their peptide conjugates: Therapeutic promise and challenge for Duchenne muscular dystrophy. Biochim. Biophys. Acta Biomembr. 2010, 1798, 2296–2303. 10.1016/j.bbamem.2010.02.012. - DOI - PubMed
- Passini M. A.; Gan L.; Wood J. A.; Yao M.; Estrella N. L.; Treleaven C. M.; Wentworth B. M.; Charleston J. S.; Rutkowski J. V.; Hanson G. J. Development of PPMO for the Treatment of Duchenne Muscular Dystrophy. Neurology 2018, 90.
- Wu B.; Moulton H. M.; Iversen P. L.; Jiang J.; Li J.; Li J.; Spurney C. F.; Sali A.; Guerron A. D.; Nagaraju K.; Doran T.; Lu P.; Xiao X.; Lu Q. L. Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modified morpholino oligomer. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 14814–14819. 10.1073/pnas.0805676105. - DOI - PMC - PubMed
- Yin H.; Moulton H. M.; Betts C.; Merritt T.; Seow Y.; Ashraf S.; Wang Q.; Boutilier J.; Wood M. J. Functional Rescue of Dystrophin-deficient mdx Mice by a Chimeric Peptide-PMO. Mol. Ther. 2010, 18, 1822–1829. 10.1038/mt.2010.151. - DOI - PMC - PubMed
-
- Egholm M.; Buchardt O.; Christensen L.; Behrens C.; Freier S. M.; Driver D. A.; Berg R. H.; Kim S. K.; Norden B.; Nielsen P. E. PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen-bonding rules. Nature 1993, 365, 566–568. 10.1038/365566a0. - DOI - PubMed
- Nielsen P.; Egholm M.; Berg R.; Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 1991, 254, 1497–1500. 10.1126/science.1962210. - DOI - PubMed
-
- Abes S.; Turner J. J.; Ivanova G. D.; Owen D.; Williams D.; Arzumanov A.; Clair P.; Gait M. J.; Lebleu B. Efficient splicing correction by PNA conjugation to an R-6-Penetratin delivery peptide. Nucleic Acids Res. 2007, 35, 4495–4502. 10.1093/nar/gkm418. - DOI - PMC - PubMed
- El-Andaloussi S.; Johansson H. J.; Lundberg P.; Langel Ü. Induction of splice correction by cell-penetrating peptide nucleic acids. J. Gene Med. 2006, 8, 1262–1273. 10.1002/jgm.950. - DOI - PubMed
- Hassane F. S.; Ivanova G. D.; Bolewska-Pedyczak E.; Abes R.; Arzumanov A. A.; Gait M. J.; Lebleu B.; Gariépy J. A Peptide-Based Dendrimer That Enhances the Splice-Redirecting Activity of PNA Conjugates in Cells. Bioconjugate Chem. 2009, 20, 1523–1530. 10.1021/bc900075p. - DOI - PubMed
- Ivanova G. D.; Arzumanov A.; Abes R.; Yin H.; Wood M. J. A.; Lebleu B.; Gait M. J. Improved cell-penetrating peptide-PNA conjugates for splicing redirection in HeLa cells and exon skipping in mdx mouse muscle. Nucleic Acids Res. 2008, 36, 6418–6428. 10.1093/nar/gkn671. - DOI - PMC - PubMed
- Koppelhus U.; Shiraishi T.; Zachar V.; Pankratova S.; Nielsen P. E. Improved cellular activity of antisense peptide nucleic acids by conjugation to a cationic peptide-lipid (CatLip) domain. Bioconjugate Chem. 2008, 19, 1526–1534. 10.1021/bc800068h. - DOI - PubMed
- Turner J. J.; Ivanova G. D.; Verbeure B.; Williams D.; Arzumanov A. A.; Abes S.; Lebleu B.; Gait M. J. Cell-penetrating peptide conjugates of peptide nucleic acids (PNA) as inhibitors of HIV-1 Tat-dependent trans-activation in cells. Nucleic Acids Res. 2005, 33, 6837–6849. 10.1093/nar/gki991. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
