Acute liver failure
- PMID: 31498101
- PMCID: PMC10836844
- DOI: 10.1016/S0140-6736(19)31894-X
Acute liver failure
Abstract
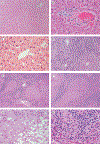
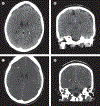
Acute liver failure is a rare and severe consequence of abrupt hepatocyte injury, and can evolve over days or weeks to a lethal outcome. A variety of insults to liver cells result in a consistent pattern of rapid-onset elevation of aminotransferases, altered mentation, and disturbed coagulation. The absence of existing liver disease distinguishes acute liver failure from decompensated cirrhosis or acute-on-chronic liver failure. Causes of acute liver failure include paracetamol toxicity, hepatic ischaemia, viral and autoimmune hepatitis, and drug-induced liver injury from prescription drugs, and herbal and dietary supplements. Diagnosis requires careful review of medications taken, and serological testing for possible viral exposure. Because of its rarity, acute liver failure has not been studied in large, randomised trials, and most treatment recommendations represent expert opinion. Improvements in management have resulted in lower mortality, although liver transplantation, used in nearly 30% of patients with acute liver failure, still provides a life-saving alternative to medical management.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
WML has received research grant support from Merck, Conatus, Intercept, Bristol-Myers Squibb, Novo Nordisk, Synlogic, Eiger, Cumberland, Exalenz Bioscience Company, Instrumentation Laboratory, and Ocera Therapeutics (now Mallinckrodt Pharmaceuticals), in the past 36 months. He has received personal fees for consulting from Novartis, Sanofi, and Genentech. RTS has received grant support from Instrumentation Laboratory, Exalenz Bioscience Company, and Ocera Therapeutics (now Mallinckrodt Pharmaceuticals), in the past 36 months.
Figures

Comment in
-
Acute liver failure.Lancet. 2020 Jun 13;395(10240):1833. doi: 10.1016/S0140-6736(20)30046-5. Lancet. 2020. PMID: 32534641 No abstract available.
References
-
- Trey C, Davidson CS. The management of fulminant hepatic failure. Prog Liver Dis 1970; 3: 282–98. - PubMed
-
- Bernal W, Jalan R, Quaglia A, Simpson K, Wendon J, Burroughs A. Acute-on-chronic liver failure. Lancet 2015; 386: 1576–87. - PubMed
-
- Wijdicks EFM. Hepatic encephalopathy. N Engl J Med 2017; 376: 186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

