Brain-First versus Gut-First Parkinson's Disease: A Hypothesis
- PMID: 31498132
- PMCID: PMC6839496
- DOI: 10.3233/JPD-191721
Brain-First versus Gut-First Parkinson's Disease: A Hypothesis
Abstract
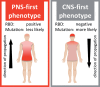
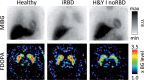
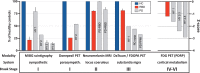
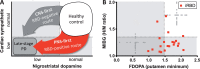
Parkinson's disease (PD) is a highly heterogeneous disorder, which probably consists of multiple subtypes. Aggregation of misfolded alpha-synuclein and propagation of these proteinacious aggregates through interconnected neural networks is believed to be a crucial pathogenetic factor. It has been hypothesized that the initial pathological alpha-synuclein aggregates originate in the enteric or peripheral nervous system (PNS) and invade the central nervous system (CNS) via retrograde vagal transport. However, evidence from neuropathological studies suggests that not all PD patients can be reconciled with this hypothesis. Importantly, a small fraction of patients do not show pathology in the dorsal motor nucleus of the vagus. Here, it is hypothesized that PD can be divided into a PNS-first and a CNS-first subtype. The former is tightly associated with REM sleep behavior disorder (RBD) during the prodromal phase and is characterized by marked autonomic damage before involvement of the dopaminergic system. In contrast, the CNS-first phenotype is most often RBD-negative during the prodromal phase and characterized by nigrostriatal dopaminergic dysfunction prior to involvement of the autonomic PNS. The existence of these subtypes is supported by in vivo imaging studies of RBD-positive and RBD-negative patient groups and by histological evidence- reviewed herein. The present proposal provides a fresh hypothesis-generating framework for future studies into the etiopathogenesis of PD and seems capable of explaining a number of discrepant findings in the neuropathological literature.
Keywords: MRI; PET; Parkinson’s disease; alpha-synuclein; autonomic nervous system; dopamine; etiology; histology; imaging; prion-like.
Conflict of interest statement
The authors have no conflict of interest to report.
Figures

References
-
- Sauerbier A, Jenner P, Todorova A, Chaudhuri KR (2016) Non motor subtypes and Parkinson’s disease. Parkinsonism Relat Disord 22(Suppl 1), S41–46. - PubMed
-
- Marras C, Chaudhuri KR (2016) Nonmotor features of Parkinson’s disease subtypes. Mov Disord 31, 1095–1102. - PubMed
-
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24, 197–211. - PubMed
-
- Braak H, Rub U, Gai WP, Del Tredici K (2003) Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna) 110, 517–536. - PubMed
-
- Svensson E, Horvath-Puho E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, Sorensen HT (2015) Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol 78, 522–529. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

