Anti-HIV potency of T-cell responses elicited by dendritic cell therapeutic vaccination
- PMID: 31498845
- PMCID: PMC6733439
- DOI: 10.1371/journal.ppat.1008011
Anti-HIV potency of T-cell responses elicited by dendritic cell therapeutic vaccination
Abstract
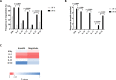
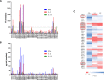
Identification and characterization of CD8+ and CD4+ T-cell epitopes elicited by HIV therapeutic vaccination is key for elucidating the nature of protective cellular responses and mechanism of the immune evasion of HIV. Here, we report the characterization of HIV-specific T-cell responses in cART (combination antiretroviral therapy) treated HIV-1 infected patients after vaccination with ex vivo-generated IFNα Dendritic Cells (DCs) loaded with LIPO-5 (HIV-1 Nef 66-97, Nef 116-145, Gag 17-35, Gag 253-284 and Pol 325-355 lipopeptides). Vaccination induced and/or expanded HIV-specific CD8+ T cells producing IFNγ, perforin, granzyme A and granzyme B, and also CD4+ T cells secreting IFNγ, IL-2 and IL-13. These responses were directed against dominant and subdominant epitopes representing all vaccine regions; Gag, Pol and Nef. Interestingly, IL-2 and IL-13 produced by CD4+ T cells were negatively correlated with the peak of viral replication following analytic treatment interruption (ATI). Epitope mapping confirmed that vaccination elicited responses against predicted T-cell epitopes, but also allowed to identify a set of 8 new HIV-1 HLA-DR-restricted CD4+ T-cell epitopes. These results may help to better design future DC therapeutic vaccines and underscore the role of vaccine-elicited CD4+ T-cell responses to achieve control of HIV replication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- UNAIDS Global HIV & AIDS statistics—2018 fact sheet. Available from: http://www.unaids.org/en/resources/fact-sheet
-
- Troya J, Bascuñana J. Safety and Tolerability: Current Challenges to Antiretroviral Therapy for the Long-Term Management of HIV Infection. AIDS Rev. 2016;18(3):127–137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

