Rare patients in routine care: Treatment and outcome in advanced papillary renal cell carcinoma in the prospective German clinical RCC-Registry
- PMID: 31498894
- PMCID: PMC7003963
- DOI: 10.1002/ijc.32671
Rare patients in routine care: Treatment and outcome in advanced papillary renal cell carcinoma in the prospective German clinical RCC-Registry
Abstract
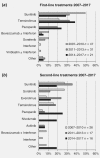
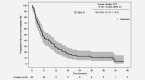
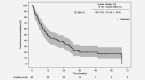
Non-clear cell renal cell carcinoma is a very rare malignancy that includes several histological subtypes. Each subtype may need to be addressed separately regarding prognosis and treatment; however, no Phase III clinical trial data exist. Thus, treatment recommendations for patients with non-clear cell metastatic RCC (mRCC) remain unclear. We present first prospective data on choice of first- and second-line treatment in routine practice and outcome of patients with papillary mRCC. From the prospective German clinical cohort study (RCC-Registry), 99 patients with papillary mRCC treated with systemic first-line therapy between December 2007 and May 2017 were included. Prospectively enrolled patients who had started first-line treatment until May 15, 2016, were included into the outcome analyses (n = 82). Treatment was similar to therapies used for clear cell mRCC and consisted of tyrosine kinase inhibitors, mechanistic target of rapamycin inhibitors and recently checkpoint inhibitors. Median progression-free survival from start of first-line treatment was 5.4 months (95% confidence interval [CI], 4.1-9.2) and median overall survival was 12.0 months (95% CI, 8.1-20.0). At data cutoff, 73% of the patients died, 6% were still observed, 12% were lost to follow-up, and 9% were alive at the end of the individual 3-year observation period. Despite the lack of prospective Phase III evidence in patients with papillary mRCC, our real-world data reveal effectiveness of systemic clear cell mRCC therapy in papillary mRCC. The prognosis seems to be inferior for papillary compared to clear cell mRCC. Further studies are needed to identify drivers of effectiveness of systemic therapy for papillary mRCC.
Keywords: cohort studies; disease management; kidney neoplasms; outcome assessment; outpatients.
© 2019 The Authors. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Figures


References
-
- Robert Koch‐Institut. Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V., eds. Krebs in Deutschland 2013/2014. Häufigkeiten und Trends. 11. Ausgabe. Berlin: Robert Koch‐Institut, 2017.
-
- Znaor A, Lortet‐Tieulent J, Laversanne M, et al. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 2015;67:519–30. - PubMed
-
- Cho E, Adami H‐O, Lindblad P. Epidemiology of renal cell cancer. Hematol Oncol Clin North Am 2011;25:651–65. - PubMed
-
- Fernández‐Pello S, Hofmann F, Tahbaz R, et al. A systematic review and meta‐analysis comparing the effectiveness and adverse effects of different systemic treatments for non‐clear cell renal cell carcinoma. Eur Urol 2017;71:426–36. - PubMed
-
- Delahunt B, Eble JN. Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol 1997;10:537–44. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

