Neural oscillations and connectivity characterizing the state of tonic experimental pain in humans
- PMID: 31498948
- PMCID: PMC7267966
- DOI: 10.1002/hbm.24784
Neural oscillations and connectivity characterizing the state of tonic experimental pain in humans
Abstract
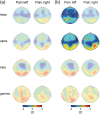
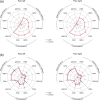
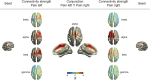
Pain is a complex phenomenon that is served by neural oscillations and connectivity involving different brain areas and frequencies. Here, we aimed to systematically and comprehensively assess the pattern of neural oscillations and connectivity characterizing the state of tonic experimental pain in humans. To this end, we applied 10-min heat pain stimuli consecutively to the right and left hand of 39 healthy participants and recorded electroencephalography. We systematically analyzed global and local measures of oscillatory brain activity, connectivity, and graph theory-based network measures during tonic pain and compared them to a nonpainful control condition. Local measures showed suppressions of oscillatory activity at alpha frequencies together with stronger connectivity at alpha and beta frequencies in sensorimotor areas during tonic pain. Furthermore, sensorimotor areas contralateral to stimulation showed significantly increased connectivity to a common area in the medial prefrontal cortex at alpha frequencies. Together, these observations indicate that the state of tonic experimental pain is associated with a sensorimotor-prefrontal network connected at alpha frequencies. These findings represent a step further toward understanding the brain mechanisms underlying long-lasting pain states in health and disease.
Keywords: brain networks; electroencephalography; functional connectivity; graph theory; neuronal oscillations; tonic pain.
© 2019 The Authors. Human Brain Mapping published by Wiley Periodicals, Inc.
Figures



References
-
- Baliki, M. N. , Chialvo, D. R. , Geha, P. Y. , Levy, R. M. , Harden, R. N. , Parrish, T. B. , & Apkarian, A. V. (2006). Chronic pain and the emotional brain: Specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. The Journal of Neuroscience, 26(47), 12165–12173. 10.1523/JNEUROSCI.3576-06.2006 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

