Characterization of stitch adhesions: Fibronectin-containing cell-cell contacts formed by fibroblasts
- PMID: 31499058
- PMCID: PMC6778521
- DOI: 10.1016/j.yexcr.2019.111616
Characterization of stitch adhesions: Fibronectin-containing cell-cell contacts formed by fibroblasts
Abstract
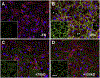
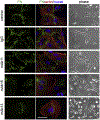
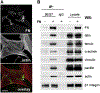
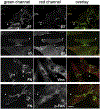
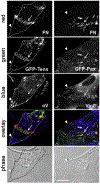
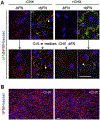
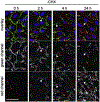
Fibronectin is a multifunctional, extracellular matrix glycoprotein that exists either as an insoluble multimeric fibrillar component of the extracellular matrix or as a soluble monomer. Cells attach to fibronectin through transmembrane integrin receptors and form a variety of cell-matrix contacts. Here we show that primary fibroblasts can use fibronectin to organize a specific cell-cell contact - "stitch adhesions." This contact is formed by short parallel fibronectin fibrils connecting adjacent cells above the level of the focal adhesions that attach the cells to the substrate. Stitch adhesions contain integrin α5β1 but not αVβ3, align with actin filament bundles, and contain talin, tensin, α-actinin, vinculin, paxillin and a phosphorylated form of focal adhesion kinase. This combination of components differs from the described constituents of the known cell adhesions. Stitch adhesions are organized when protein synthesis and secretion are inhibited by cycloheximide and exogenous fibronectin is provided to the cells. The adhesion stitches described here provide an attractive model system for studying fibronectin fibrillogenesis and the mechanisms governing the formation of cellular adhesions.
Keywords: Adhesion; Cell-cell contacts; Cycloheximide; Fibronectin; Human fibroblasts.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures


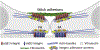
Similar articles
-
Dissecting focal adhesions in cells differentially expressing calreticulin: a microscopy study.Biol Cell. 2007 Jul;99(7):389-402. doi: 10.1042/BC20060105. Biol Cell. 2007. PMID: 17373910
-
The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis.J Cell Biol. 2002 Dec 23;159(6):1071-86. doi: 10.1083/jcb.200205014. Epub 2002 Dec 16. J Cell Biol. 2002. PMID: 12486108 Free PMC article.
-
FAK-independent alphavbeta3 integrin-EGFR complexes rescue from anoikis matrix-defective fibroblasts.Biochim Biophys Acta. 2008 Jun;1783(6):1177-88. doi: 10.1016/j.bbamcr.2008.03.003. Epub 2008 Mar 20. Biochim Biophys Acta. 2008. PMID: 18405669
-
Integrating actin dynamics, mechanotransduction and integrin activation: the multiple functions of actin binding proteins in focal adhesions.Eur J Cell Biol. 2013 Oct-Nov;92(10-11):339-48. doi: 10.1016/j.ejcb.2013.10.009. Epub 2013 Nov 4. Eur J Cell Biol. 2013. PMID: 24252517 Review.
-
Molecular complexity and dynamics of cell-matrix adhesions.J Cell Sci. 2001 Oct;114(Pt 20):3583-90. doi: 10.1242/jcs.114.20.3583. J Cell Sci. 2001. PMID: 11707510 Review.
Cited by
-
USP10 Promotes Fibronectin Recycling, Secretion, and Organization.Invest Ophthalmol Vis Sci. 2021 Oct 4;62(13):15. doi: 10.1167/iovs.62.13.15. Invest Ophthalmol Vis Sci. 2021. PMID: 34665194 Free PMC article.
-
The formin DAAM1 regulates the deubiquitinase activity of USP10 and integrin homeostasis.Eur J Cell Biol. 2023 Dec;102(4):151347. doi: 10.1016/j.ejcb.2023.151347. Epub 2023 Aug 5. Eur J Cell Biol. 2023. PMID: 37562219 Free PMC article.
-
Meaningful connections: Interrogating the role of physical fibroblast cell-cell communication in cancer.Adv Cancer Res. 2022;154:141-168. doi: 10.1016/bs.acr.2022.01.004. Epub 2022 Feb 24. Adv Cancer Res. 2022. PMID: 35459467 Free PMC article.
-
Compressive stress triggers fibroblasts spreading over cancer cells to generate carcinoma in situ organization.Commun Biol. 2024 Feb 15;7(1):184. doi: 10.1038/s42003-024-05883-6. Commun Biol. 2024. PMID: 38360973 Free PMC article.
-
Cell biomechanics on muscle atrophy: from intricate mechanisms to therapeutic frontiers.Ann Med. 2025 Dec;57(1):2540598. doi: 10.1080/07853890.2025.2540598. Epub 2025 Aug 1. Ann Med. 2025. PMID: 40747836 Free PMC article. Review.
References
-
- Hynes RO, Fibronectins. New York: Springer-Verlag, 1990. http://www.springer.com/la/book/9781461279402
-
- Pankov R, Yamada KM, Fibronectin at a glance, J. Cell Sci. 115 (2002) 3861–3863. http://jcs.biologists.org/content/115/20/3861.long - PubMed
-
- Singh P, Carraher C, Schwarzbauer JE, Assembly of fibronectin extracellular matrix, Annu. Rev. Cell Dev. Biol. 26 (2010) 397–419. https://www.annualreviews.org/doi/abs/10.1146/annurev-cellbio-100109-104020 - DOI - PMC - PubMed
-
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO, Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin, Development, 119 (1993) 1079–1091. http://dev.biologists.org/content/119/4/1079.short - PubMed
-
- Plow EF, Haas TA, Zhang L, Loftus J, Smith JW, Ligand binding to integrins, J. Biol. Chem. 275 (2000) 21785–21788. http://www.jbc.org/content/275/29/21785.short - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

