The clinical role of VeriStrat testing in patients with advanced non-small cell lung cancer considered unfit for first-line platinum-based chemotherapy
- PMID: 31499384
- PMCID: PMC6859789
- DOI: 10.1016/j.ejca.2019.07.025
The clinical role of VeriStrat testing in patients with advanced non-small cell lung cancer considered unfit for first-line platinum-based chemotherapy
Abstract
Purpose: We previously demonstrated that the median survival of patients with poor prognosis non-small cell lung cancer (NSCLC) considered unfit for first-line platinum chemotherapy was <4 months. We evaluated whether VeriStrat could be used as a prognostic or predictive biomarker in this population.
Experimental design: We conducted a randomised double-blind trial among patients with untreated advanced NSCLC considered unfit for platinum chemotherapy because of poor performance status (PS) or multiple comorbidities. All patients received active supportive care (ASC) and were treated with either oral erlotinib or placebo daily. Five hundred twenty-seven patients had plasma samples for VeriStrat classification: good (VeriStrat Good [VSG]) or poor (VeriStrat Poor [VSP]). Main end-point was overall survival.
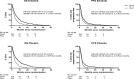
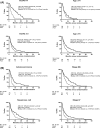
Results: Fifty-five percent patients had VSG, and 83% had Eastern Cooperative Oncology Group (ECOG) 2-3 at baseline. VeriStrat was strongly associated with survival. Among patients managed with ASC only, the adjusted hazard ratio (HR) was 0.54 (p < 0.001) for VSG versus VSP. The association was consistent across patient factors: HR = 0.25 (p = 0.004) and HR = 0.56 (p < 0.001) for ECOG 0-1 and 2-3, respectively, HR = 0.49 (0070 < 0.001) for age≥75 years and HR = 0.59 (p = 0.007) for stage IV. Several ECOG 2-3 patients had long survival: 2-year survival was 8% for VSG patients who had ASC, compared with 0% for VSP. VeriStrat status did not predict benefit from erlotinib treatment because the HRs for erlotinib versus placebo were similar between VSG and VSP patients.
Conclusions: VeriStrat was not a predictive marker for survival when considering first-line erlotinib for patients with NSCLC who had poor PS and were not recommended for platinum doublet therapies. However, VeriStrat was an independent prognostic marker of survival. It represents an objective measurement that could be considered alongside other patient factors to provide a more refined assessment of prognosis for this particular patient group. VSG patients could be selected for treatment trials because of better survival, while VSP patients can continue to be treated conservatively or offered trials of less toxic agents.
Trial registration isrctn number: ISRCTN02370070.
Keywords: Active supportive care; Biomarker; Non-small cell lung cancer; Poor performance ECOG 2&3; Predictive; Prognostic; Proteonomic; VeriStrat.
Crown Copyright © 2019. Published by Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Retrospective Assessment of a Serum Proteomic Test in a Phase III Study Comparing Erlotinib plus Placebo with Erlotinib plus Tivantinib (MARQUEE) in Previously Treated Patients with Advanced Non-Small Cell Lung Cancer.Oncologist. 2019 Jun;24(6):e251-e259. doi: 10.1634/theoncologist.2018-0089. Epub 2018 Aug 23. Oncologist. 2019. PMID: 30139835 Free PMC article. Clinical Trial.
-
The serum-based VeriStrat® test is associated with proinflammatory reactants and clinical outcome in non-small cell lung cancer patients.BMC Cancer. 2018 Mar 20;18(1):310. doi: 10.1186/s12885-018-4193-0. BMC Cancer. 2018. PMID: 29558888 Free PMC article.
-
Randomized Phase III Trial of Erlotinib versus Docetaxel in Patients with Advanced Squamous Cell Non-Small Cell Lung Cancer Failing First-Line Platinum-Based Doublet Chemotherapy Stratified by VeriStrat Good versus VeriStrat Poor. The European Thoracic Oncology Platform (ETOP) EMPHASIS-lung Trial.J Thorac Oncol. 2017 Apr;12(4):752-762. doi: 10.1016/j.jtho.2016.12.017. Epub 2016 Dec 23. J Thorac Oncol. 2017. PMID: 28017787 Clinical Trial.
-
Prognostic performance of proteomic testing in advanced non-small cell lung cancer: a systematic literature review and meta-analysis.Curr Med Res Opin. 2020 Sep;36(9):1497-1505. doi: 10.1080/03007995.2020.1790346. Epub 2020 Jul 23. Curr Med Res Opin. 2020. PMID: 32615813
-
Poor-Performance Status Assessment of Patients with Non-small Cell Lung Cancer Remains Vague and Blurred in the Immunotherapy Era.Curr Oncol Rep. 2019 Nov 25;21(12):107. doi: 10.1007/s11912-019-0852-9. Curr Oncol Rep. 2019. PMID: 31768759 Review.
Cited by
-
Molecular and translational biology of the blood-based VeriStrat® proteomic test used in cancer immunotherapy treatment guidance.J Mass Spectrom Adv Clin Lab. 2023 Nov 20;30:51-60. doi: 10.1016/j.jmsacl.2023.11.001. eCollection 2023 Nov. J Mass Spectrom Adv Clin Lab. 2023. PMID: 38074293 Free PMC article.
-
Influence of Smoking Habits on the Efficacy of EGFR-TKI Therapy in Patients with Advanced NSCLC: A Systematic Review and Meta-Analysis.Clin Med Insights Oncol. 2023 Dec 12;17:11795549231215968. doi: 10.1177/11795549231215968. eCollection 2023. Clin Med Insights Oncol. 2023. PMID: 38107371 Free PMC article.
-
Mass Spectrometry-Based Multivariate Proteomic Tests for Prediction of Outcomes on Immune Checkpoint Blockade Therapy: The Modern Analytical Approach.Int J Mol Sci. 2020 Jan 28;21(3):838. doi: 10.3390/ijms21030838. Int J Mol Sci. 2020. PMID: 32012941 Free PMC article. Review.
-
Blood serum amyloid A as potential biomarker of pembrolizumab efficacy for patients affected by advanced non-small cell lung cancer overexpressing PD-L1: results of the exploratory "FoRECATT" study.Cancer Immunol Immunother. 2021 Jun;70(6):1583-1592. doi: 10.1007/s00262-020-02788-1. Epub 2020 Nov 24. Cancer Immunol Immunother. 2021. PMID: 33231726 Free PMC article. Clinical Trial.
References
-
- Shepherd F.A., Rodrigues Pereira J., Ciuleanu T. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–132. - PubMed
-
- Kawaguchi T., Ando M., Asami K. Randomized phase III trial of erlotinib versus docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: docetaxel and Erlotinib Lung Cancer Trial (DELTA) J Clin Oncol. 2014;32(18):1902–1908. - PubMed
-
- Garassino M.C., Martelli O., Broggini M. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol. 2013;14(10):981–988. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

