Oral non-steroidal anti-inflammatory drug therapy for lung disease in cystic fibrosis
- PMID: 31499593
- PMCID: PMC6733592
- DOI: 10.1002/14651858.CD001505.pub5
Oral non-steroidal anti-inflammatory drug therapy for lung disease in cystic fibrosis
Abstract
Background: Progressive lung damage causes most deaths in cystic fibrosis. Non-steroidal anti-inflammatory drugs (such as ibuprofen) may prevent progressive pulmonary deterioration and morbidity in cystic fibrosis. This is an update of a previously published review.
Objectives: To assess the effectiveness of treatment with oral non-steroidal anti-inflammatory drugs in cystic fibrosis.
Search methods: We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register comprising references identified from comprehensive electronic database searches, hand searches of relevant journals and abstract books of conference proceedings. We contacted manufacturers of non-steroidal anti-inflammatory drugs and searched online trials registries.Latest search of the Group's Trials Register: 21 November 2018.
Selection criteria: Randomized controlled trials comparing oral non-steroidal anti-inflammatory drugs, at any dose for at least two months, to placebo in people with cystic fibrosis.
Data collection and analysis: Two authors independently assessed trials for inclusion the review and their potential risk of bias. Two authors independently rated the quality of the evidence for each outcome using the GRADE guidelines.
Main results: The searches identified 17 trials; four are included (287 participants aged five to 39 years; maximum follow-up of four years) and one is currently awaiting classification pending publication of the full trial report and two are ongoing. Three trials compared ibuprofen to placebo (two from the same center with some of the same participants); one trial assessed piroxicam versus placebo.The three ibuprofen trials were deemed to have good or adequate methodological quality, but used various outcomes and summary measures. Reviewers considered measures of lung function, nutritional status, radiological assessment of pulmonary involvement, intravenous antibiotic usage, hospital admissions, survival and adverse effects. Combined data from the two largest ibuprofen trials showed a lower annual rate of decline for lung function, % predicted forced expiratory volume in one second (FEV1), mean difference (MD) 1.32 (95% confidence interval (CI) 0.21 to 2.42) (moderate-quality evidence); forced vital capacity (FVC), MD 1.27 (95% CI 0.26 to 2.28) (moderate-quality evidence); forced expiratory flow (FEF25%-75%), MD 1.80 (95% CI 0.15 to 3.45). The post hoc analysis of data from two trials split by age showed a slower rate of annual decline of FEV1 % predicted and FVC in the ibuprofen group in younger children, MD 1.41% (95% CI 0.03 to 2.80) (moderate-quality evidence) and MD 1.32% (95% CI 0.04 to 2.60) (moderate-quality evidence) respectively. Data from four trials demonstrated the proportion of participants with at least one hospitalization may be slightly lower in the ibuprofen group compared to placebo, Peto odds ratio 0.61 (95% CI 0.37 to 1.01) (moderate-quality evidence). In one trial, long-term use of high-dose ibuprofen was associated with reduced intravenous antibiotic usage, improved nutritional and radiological pulmonary status. No major adverse effects were reported, but the power of the trials to identify clinically important differences in the incidence of adverse effects was low.We did not have any concerns with regards to risk of bias for the trial comparing piroxicam to placebo. However, the trial did not report many data in a form that we could analyze in this review. No data were available for the review's primary outcome of lung function; available data for hospital admissions showed no difference between the groups. No analyzable data were available for any other review outcome.
Authors' conclusions: High-dose ibuprofen can slow the progression of lung disease in people with cystic fibrosis, especially in children, which suggests that strategies to modulate lung inflammation can be beneficial for people with cystic fibrosis.
Conflict of interest statement
Dr Larry Lands was the lead investigator in the Trans‐Canadian Trial (Lands 2007). He is also the chief medical advisor for a phase II trial currently underway of fenretinide for CF adults as an anti‐inflammatory therapy; this drug is not eligible for inclusion in this review.
Dr Sanja Stanojevic declares no known conflict of interest.
Figures









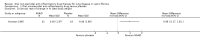






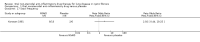







Update of
-
Oral non-steroidal anti-inflammatory drug therapy for lung disease in cystic fibrosis.Cochrane Database Syst Rev. 2016 Apr 7;4:CD001505. doi: 10.1002/14651858.CD001505.pub4. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2019 Sep 09;9:CD001505. doi: 10.1002/14651858.CD001505.pub5. PMID: 27055154 Updated.
References
References to studies included in this review
Konstan 1991 {published data only}
-
- Konstan MW, Hoppel CL, Chai B, Davis PB. Ibuprofen in children with cystic fibrosis: pharmacokinetics and adverse effects. Journal of Pediatrics 1991;118(6):956‐64. [CFGD Register: IB5] - PubMed
Konstan 1995 {published data only}
-
- Konstan MW. Systemic anti‐inflammatory treatment NSAID. Proceedings of the 20th European Cystic Fibrosis Conference; 1995 June 18‐21; Brussels, Belgium. 1995:L51. [CFGD Register: IB10a]
-
- Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high‐dose ibuprofen in patients with cystic fibrosis. New England Journal of Medicine 1995;332(13):848‐54. [CFGD Register: IB10c] - PubMed
-
- Konstan MW, Davis PB, Byard PJ, Hoppel CJ. Results of a four‐year, randomized, placebo‐controlled, double‐blind trial of high‐dose ibuprofen in CF patients with mild lung disease [abstract]. Pediatric Pulmonology 1994;Suppl 10:S6.4. [CFGD Register: IB10b]
Lands 2007 {published data only}
-
- Lands LC, Corey M, Milner R, Kilcullen A, Cantin AM. High dose ibuprofen in CF children: the Trans‐Canadian trial. Pediatric Pulmonology 2002;34(Suppl 24):276. [CFGD Register: IB38a]
-
- Lands LC, Milner R, Cantain AM, Manson D, Corey M. High‐dose Ibuprofen in Cystic Fibrosis: Canadian Safety and Effectiveness Trial. Journal of Pediatrics 2007;151(3):249‐54. [CFGD Register: IB38b] - PubMed
Sordelli 1994 {published data only}
-
- Sordelli DO, Macri CN, Maillie AJ. A study on the effect of Piroxicam (PIR) treatment to prevent lung damage in CF patients with Pseudomonas aeruginosa (Psa) pneumonia. Pediatric Pulmonology 1990;Suppl 5:215. [CFGD Register: IB6b]
-
- Sordelli DO, Macri CN, Maillie AJ, Cerquetti MC. A preliminary study of the effect of anti‐inflammatory treatment in cystic fibrosis patients with pseudomonas aeruginosa lung infection. International Journal of Immunopathology and Pharmacology 1994;7(2):109‐17. [CFGD Register: IB6b]
References to studies excluded from this review
Chmiel 2007 {published data only}
-
- Chmiel JF, Konstan MW, Accurso FJ, Lymp J, Mayer‐Hamblett N, VanDevanter DR, et al. Use of ibuprofen to assess inflammatory biomarkers in induced sputum: Implications for clinical trials in cystic fibrosis. Journal of Cystic Fibrosis 2015;14(6):720‐6. [CFGD Register: PI218b] - PubMed
-
- Chmiel JF, Konstan MW, Lymp J, Mayer‐Hamblett N, Hilliard KA, Accurso FJ, et al. Assessment of induced sputum as a tool to evaluate anti‐inflammatory agents in CF. Pediatric Pulmonology 2007;42 Suppl 30:228. [CFGD Register: PI218a]
Gonzalez 2018 {published data only}
-
- Gonzalez V, Siegler N, Crandall R, Mckie KT, Forseen C, Rodriguez‐Miguelez P, et al. Acute sildenafil treatment improves exercise capacity in patients with cystic fibrosis. FASEB Journal. Experimental Biology Abstracts 2018;32(Supplement 1):no pagination. [Abstract No.: 853.5; CFGD Register: IB124]
Hunt 2017 {published data only}
-
- Hunt K, Clair C, Curran‐Everett D, Solomon GM, Saavedra MT, Nick JA, et al. CFTR effects of oral sildenafil in combination with lumacaftor/ivacaftor in adults with CF. Pediatric Pulmonology 2017;52 Suppl 47:322. [CFGD Register: IB117]
Kovaleva 2000 {published data only}
-
- Kovaleva LF, Guembitskaia TE, Gorbenko IA, Aleshin YuN. Combined systemic enzymotherapy and nebulizer therapy in the management of cystic fibrosis (CF) patients. Proceedings of the 13th International Cystic Fibrosis Congress; 2000 June 4‐8; Stockholm, Sweden. 2000:155. [CFGD Register: IB91]
Noritake 1982 {published data only}
-
- Noritake D, Hen J, Dolan TF. Effects of aspirin on pulmonary function in patients with cystic fibrosis. Proceedings of the 22nd Annual Meeting Cystic Fibrosis Club Abstracts; 1981 May 1; San Francisco, California. 1981:144. [CFGD Register: IB4b]
-
- Noritake DT, Hen J, Leo L, Dolan TF. The influence of aspirin on lung function in cystic fibrosis. Connecticut Medicine 1982;46:574‐6. [CFGD Register: IB4a] - PubMed
Shmarina 2004 {published and unpublished data}
-
- Pukhalsky AL, Shmarina GV, Kapranov NI, Kakorovtseva SN, Pukhalskaya D, Kashirskaja NJ. Anti‐inflammatory and immunomodulating effects of clarithromycin in patients with cystic fibrosis lung disease. Mediator of Inflammation 2004;13(2):111‐7. [CENTRAL: 997725; CFGD Register: IB44c; CRS: 5500125000000705; PUBMED: 15203552] - PMC - PubMed
-
- Pukhalsky AL, Shmarina GV, Kaproanov NI, Kashirskaja NJ, Kokarovtseva SN, Shabalova LA. Increase of the sputumneutrophil elastase activity in a paradoxical effect of the successful lung disease treatment in cystic fibrosis. Pediatric Pulmonology 2001;32 (Suppl 22):274. [CFGD Register: IB44b]
-
- Shmarina GV, Pukhalsky AL, Kashirskaja NJ. Anti‐inflammatory therapy in cystic fibrosis: a comparative study in the treatment with nimesulide (selective cyclooxygenase‐2 inhibitor) and clarithromycin (14‐membrered ring macrolide antibiotic). European Respiratory Journal. 2004; Vol. Suppl 48:P3758. [CFGD Register: IB44a]
Springman 2014 {published data only}
-
- Ahuja S, Springman EB, Grosswald R, Philpot E, MacGregor G, Horsley A, et al. CTX‐ 4430 in a phase 1 clinical trial in cystic fibrosis patients. Pediatric Pulmonology 2015;50 Suppl 41:299. [Abstract no. : 287; CENTRAL: 1092202; CFGD Register: IB107d; CRS: 5500135000001391]
-
- Springman E, Bhatt L, Grosswald R, Philpot E. Pharmacokinetic and pharmacodynamic profile of CTX ‐4430 in two phase 1 studies. Journal of Cystic Fibrosis 2015;14 Suppl 1:S90. [Abstract no.: 127; CENTRAL: 1077210; CFGD Register: IB107c; CRS: 5500135000001299]
-
- Springman E, Grosswald R, Philpot E, MacGregor G, Horsley A, Bilton D, et al. A phase 1 clinical study of CTX‐4430 in cystic fibrosis patients. Inflammation Research 2015;64(2 Suppl 1):S206‐7. [Abstract no.: B261; CENTRAL: 1107950; CFGD Register: IB107e; CRS: 5500050000000292; EMBASE: 71973697]
-
- Springman E, Grosswald R, Philpot E, MacGregor G, Horsley A, Bilton D, et al. A phase 1 clinical study of CTX‐4430 in cystic fibrosis patients. Journal of Cystic Fibrosis 2015;14 Suppl 1:S90. [Abstract no.: 126; CENTRAL: 1077211; CFGD Register: IB107b; CRS: 5500135000001300]
-
- Springman EB, Grosswald R, Philpot E, MacGregor G, Horsley A, Bilton D, et al. A phase 1 clinical study of CTX‐4430 in cystic fibrosis patients. Pediatric Pulmonology 2014;49 Suppl 38:311. [Abstract no.: 267; CENTRAL: 1012389; CFGD Register: IB107a; CRS: 5500131000000163]
Taylor‐Cousar 2017 {published data only}
-
- Taylor‐Cousar JL, Hunt KC, Clair C, Everett DC, Nick JA, Saavedra MT, Abman SH. A randomised controlled pilot study of sildenafil to treat exercise intolerance in moderate to severe cystic fibrosis. Journal of Cystic Fibrosis 2018;17 Suppl 3:S26. [Abstract no.: WS14.5; CFGD Register: IB121a]
-
- Tayor‐Cousar JL, Hunt KC, St. Clair C, Everett D, Fenster B, Nick JA, et al. Sildenafil improves cardiac performance, exercise intolerance and quality of life in moderate to severe cf lung disease. Pediatric Pulmonology 2018;53 Suppl 2:226‐7. [Abstract no.: 212; CFGD Register: IB121b]
Vargas 2017 {published data only}
-
- Vargas MH, Del‐Razo‐Rodriguez R, Lopez‐Garcia A, Lezana‐Fernandez JL, Chavez J, Furuya MEY, et al. Effect of oral glycine on the clinical, spirometric and inflammatory status in subjects with cystic fibrosis: a pilot randomized trial. BMC Pulmonary Medicine 2017;17(1):206. [CFGD Register: IB125a; DOI: 10.1186/s12890-017-0528-x] - DOI - PMC - PubMed
Zeitlin 2017 {published data only}
-
- Callahan KA, Diener‐West M, Schoeberlein C, Boyle MP, Zeitlin PL. Preliminary safety profile of digitoxin to treat CF. Pediatric Pulmonology 2014;49(Suppl 38):293. [Abstract no.: 219; CFGD Register: IB109b]
-
- Lee S, Walker D, Zeitlin P. Nasal microarray analysis in CF subjects taking digitoxin. Pediatric Pulmonology 2015;50 Suppl 41:292. [Abstract no.: 267; CFGD Register: IB109c]
-
- Zeitlin P, Diener‐West M, Callahan KA, Pollard B, Lechtzin N, Boyle MP. Phase II study of digitoxin for CF. Pediatric Pulmonology 2015;50 Suppl 41:268. [Abstract no.: 207; CFGD Register: IB109d]
-
- Zeitlin PL. Phase II study of digitoxin to treat cystic fibrosis. clinicaltrials.gov/ct2/show/NCT00782288 (first posted 31 October 2008). [CFGD Register: IB109a]
-
- Zeitlin PL, Diener‐West M, Callahan KA, Lee S, Talbot CC Jr, Pollard B, et al. Digitoxin for airway inflammation in cystic fibrosis: preliminary assessment of safety, pharmacokinetics, and dose finding. Annals of the American Thoracic Society 2017;14(2):220‐9. [CFGD Register: IB109e; ] - PMC - PubMed
References to studies awaiting assessment
EMPIRE 2018 {published data only}
-
- Elborn J, Taylor‐Cousar JL, Rowe SM, Grosswald R, Mershon J, Springman E, et al. A phase 2 trial (EMPIRE CF) of a novel anti‐inflammatory molecule, acebilustat, in patients with cystic fibrosis. Pediatric Pulmonology 2018;53 Suppl 2:227‐8. [Abstract no.: 214; CFGD Register: IB122c]
-
- Elborn JS, Ahuja S, Mershon J, Springman E, Rowe SM. Demographics of patients in a phase 2 trial of acebilustat in patients with cystic fibrosis (EMPIRE CF). Journal of Cystic Fibrosis 2018;17(Suppl 3):S27. [CFGD Register: IB122a]
-
- Elborn JS, Ahuja S, Springman E, Mershon J, Grosswald R, Rowe SM. EMPIRE‐CF: a phase II randomized placebo‐controlled trial of once‐daily, oral acebilustat in adult patients with cystic fibrosis ‐ Study design and patient demographics. Contemporary Clinical Trials 2018;72:86‐94. [CFGD Register: IB122b] - PubMed
References to ongoing studies
APPLAUD 2017 {unpublished data only}
-
- NCT03265288. Study of LAU‐7b in the Treatment of Cystic Fibrosis in Adults (APPLAUD). clinicaltrials.gov/ct2/show/NCT03265288 (first posted 29 August 2017).
NCT03451045 {published data only}
-
- NCT03451045. Trial to evaluate efficacy and safety of lenabasum in cystic fibrosis. clinicaltrials.gov/ct2/show/NCT03451045 (first posted 01 March 2018).
Additional references
Bertenshaw 2007
-
- Bertenshaw C, Watson A, Lewis S, Smyth A. A survey of acute renal failure in cystic fibrosis patients in the United Kingdom. http://thorax.bmj.com/ (accessed 30 January 2007).
Chmiel 2002
-
- Chmiel JF, Berger M, Konstan MW. The role of inflammation in the pathophysiology of CF lung disease. Clinical Reviews in Allergy & Immunology 2002;23(1):5‐27. - PubMed
Farrell 2018
-
- Farrell P, Férec C, Macek M, Frischer T, Renner S, Riss K, et al. Estimating the age of p.(Phe508del) with family studies of geographically distinct European populations and the early spread of cystic fibrosis. European Journal of Human Genetics 2018;26(12):1832‐9. [DOI: 10.1038/s41431-018-0234-z] - DOI - PMC - PubMed
Gibson 2003
-
- Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. American Journal Respiratory and Critical Care Medicine 2003;168(8):918‐51. - PubMed
Higgins 2003
Konstan 1990
-
- Konstan MW, Vargo KM, Davis PB. Ibuprofen attenuates the inflammatory response to Pseudomonas aeruginosa in a rat model of chronic pulmonary infection. American Review of Respiratory and Critical Care Medicine 1990;141(1):186‐92. - PubMed
Konstan 1997
-
- Konstan MW, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatric Pulmonology 1997;24(2):137‐42. - PubMed
Konstan 1999
-
- Konstan MW. Personal Communication 1999.
Konstan 2003
-
- Konstan MW, Krenicky JE, Finney MR, Kirchner HL, Hilliard KA, Hilliard JB, et al. Effect of ibuprofen on neutrophil migration in vivo in cystic fibrosis and healthy subjects. Journal of Pharmacology and Experimental Therapeutics 2003;306(3):1086‐91. - PubMed
Konstan 2007
Konstan 2018
-
- Konstan MW, VanDevanter DR, Sawicki GS, Pasta DJ, Foreman AJ, Neiman EA, et al. Association of high‐dose ibuprofen use, lung function decline and long‐term survival in children with cystic fibrosis. Annals of the American Thoracic Society 2018;15(4):485‐93. - PubMed
Kovesi 1998
-
- Kovesi TA, Swartz R, MacDonald N. Transient renal failure due to simultaneous ibuprofen and aminoglycoside therapy in children with cystic fibrosis (letter). New England Journal of Medicine 1998;338(1):65‐6. - PubMed
Li 2008
-
- Li J, Xiang YY, Ye L, Tsui LC, Macdonald JF, Hu J, et al. Nonsteroidal anti‐inflammatory drugs upregulate function of wild‐type and mutant CFTR. European Respiratory Journal 2008;32(2):334‐43. - PubMed
Rosenstein 1998
-
- Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation Consensus Panel. Journal of Pediatrics 1998;132(4):589‐95. - PubMed
Southern 2004
Wheeler 1984
-
- Wheeler WB, Williams RN, Matthews WJ, Colten HR. Progression of cystic fibrosis lung disease as a function of serum immunoglobulin G levels: a 5‐year longitudinal study. Journal of Pediatrics 1984;104(5):685‐99. - PubMed
References to other published versions of this review
Dezateux 1999
Lands 2007
Lands 2009
Lands 2013
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

