Viscosity of Plasma as a Key Factor in Assessment of Extracellular Vesicles by Light Scattering
- PMID: 31500151
- PMCID: PMC6769602
- DOI: 10.3390/cells8091046
Viscosity of Plasma as a Key Factor in Assessment of Extracellular Vesicles by Light Scattering
Abstract
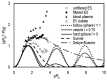
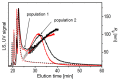
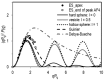
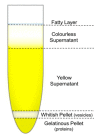
Extracellular vesicles (EVs) isolated from biological samples are a promising material for use in medicine and technology. However, the assessment methods that would yield repeatable concentrations, sizes and compositions of the harvested material are missing. A plausible model for the description of EV isolates has not been developed. Furthermore, the identity and genesis of EVs are still obscure and the relevant parameters have not yet been identified. The purpose of this work is to better understand the mechanisms taking place during harvesting of EVs, in particular the role of viscosity of EV suspension. The EVs were harvested from blood plasma by repeated centrifugation and washing of samples. Their size and shape were assessed by using a combination of static and dynamic light scattering. The average shape parameter of the assessed particles was found to be ρ ~ 1 (0.94-1.1 in exosome standards and 0.7-1.2 in blood plasma and EV isolates), pertaining to spherical shells (spherical vesicles). This study has estimated the value of the viscosity coefficient of the medium in blood plasma to be 1.2 mPa/s. It can be concluded that light scattering could be a plausible method for the assessment of EVs upon considering that EVs are a dynamic material with a transient identity.
Keywords: blood plasma; dynamic light scattering; exosomes; extracellular vesicles; shape characterization; static light scattering; viscosity of blood plasma.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Simpson R.J., Mathivanan S. Extracellular Microvesicles: The Need for Internationally Recognised Nomenclature and Stringent Purification Criteria. J. Proteomics Bioinform. 2012 doi: 10.4172/jpb.10000e10. - DOI
-
- Černe K., Kobal B. Chapter Eight-Implications of Microvesicle and Cell Surface Protein Shedding for Biomarker Studies, Cancerogenesis, and Therapeutic Target Discovery in Ovarian Cancer. In: Aleš I., editor. Advances in Planar Lipid Bilayers and Liposomes. Volume 16. Academic Press; Cambridge, MA, USA: 2012. pp. 239–274.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

