Structure and Dynamics of Thermosensitive pDNA Polyplexes Studied by Time-Resolved Fluorescence Spectroscopy
- PMID: 31500418
- PMCID: PMC6961130
- DOI: 10.1021/acs.biomac.9b00896
Structure and Dynamics of Thermosensitive pDNA Polyplexes Studied by Time-Resolved Fluorescence Spectroscopy
Abstract
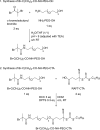
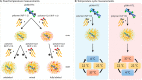
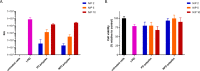
Combining multiple stimuli-responsive functionalities into the polymer design is an attractive approach to improve nucleic acid delivery. However, more in-depth fundamental understanding how the multiple functionalities in the polymer structures are influencing polyplex formation and stability is essential for the rational development of such delivery systems. Therefore, in this study the structure and dynamics of thermosensitive polyplexes were investigated by tracking the behavior of labeled plasmid DNA (pDNA) and polymer with time-resolved fluorescence spectroscopy using fluorescence resonance energy transfer (FRET). The successful synthesis of a heterofunctional poly(ethylene glycol) (PEG) macroinitiator containing both an atom transfer radical polymerization (ATRP) and reversible addition-fragmentation chain-transfer (RAFT) initiator is reported. The use of this novel PEG macroinitiator allows for the controlled polymerization of cationic and thermosensitive linear triblock copolymers and labeling of the chain-end with a fluorescent dye by maleimide-thiol chemistry. The polymers consisted of a thermosensitive poly(N-isopropylacrylamide) (PNIPAM, N), hydrophilic PEG (P), and cationic poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA, D) block, further referred to as NPD. Polymer block D chain-ends were labeled with Cy3, while pDNA was labeled with FITC. The thermosensitive NPD polymers were used to prepare pDNA polyplexes, and the effect of the N/P charge ratio, temperature, and composition of the triblock copolymer on the polyplex properties were investigated, taking nonthermosensitive PD polymers as the control. FRET was observed both at 4 and 37 °C, indicating that the introduction of the thermosensitive PNIPAM block did not compromise the polyplex structure even above the polymer's cloud point. Furthermore, FRET results showed that the NPD- and PD-based polyplexes have a less dense core compared to polyplexes based on cationic homopolymers (such as PEI) as reported before. The polyplexes showed to have a dynamic character meaning that the polymer chains can exchange between the polyplex core and shell. Mobility of the polymers allow their uniform redistribution within the polyplex and this feature has been reported to be favorable in the context of pDNA release and subsequent improved transfection efficiency, compared to nondynamic formulations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








Similar articles
-
Local release of siRNA using polyplex-loaded thermosensitive hydrogels.Nanoscale. 2020 May 14;12(18):10347-10360. doi: 10.1039/d0nr03147j. Nanoscale. 2020. PMID: 32369076
-
Effects of the incorporation of a hydrophobic middle block into a PEG-polycation diblock copolymer on the physicochemical and cell interaction properties of the polymer-DNA complexes.Biomacromolecules. 2008 Nov;9(11):3294-307. doi: 10.1021/bm800876v. Epub 2008 Oct 23. Biomacromolecules. 2008. PMID: 18942877 Free PMC article.
-
Reversibly shielded DNA polyplexes based on bioreducible PDMAEMA-SS-PEG-SS-PDMAEMA triblock copolymers mediate markedly enhanced nonviral gene transfection.Biomacromolecules. 2012 Mar 12;13(3):769-78. doi: 10.1021/bm201693j. Epub 2012 Feb 10. Biomacromolecules. 2012. PMID: 22277017
-
Poly(2-isopropenyl-2-oxazoline) as a Versatile Functional Polymer for Biomedical Applications.Polymers (Basel). 2024 Jun 14;16(12):1708. doi: 10.3390/polym16121708. Polymers (Basel). 2024. PMID: 38932057 Free PMC article. Review.
-
Block Copolymer Synthesis by the Combination of Living Cationic Polymerization and Other Polymerization Methods.Front Chem. 2021 Jun 28;9:644547. doi: 10.3389/fchem.2021.644547. eCollection 2021. Front Chem. 2021. PMID: 34262892 Free PMC article. Review.
Cited by
-
Self-Healing Thermosensitive Hydrogel for Sustained Release of Dexamethasone for Ocular Therapy.ACS Polym Au. 2022 Nov 3;3(1):118-131. doi: 10.1021/acspolymersau.2c00038. eCollection 2023 Feb 8. ACS Polym Au. 2022. PMID: 36785837 Free PMC article.
-
Effect of Polyplex Size on Penetration into Tumor Spheroids.Mol Pharm. 2023 Nov 6;20(11):5515-5531. doi: 10.1021/acs.molpharmaceut.3c00397. Epub 2023 Oct 9. Mol Pharm. 2023. PMID: 37811785 Free PMC article.
-
Progress of cationic gene delivery reagents for non-viral vector.Appl Microbiol Biotechnol. 2021 Jan;105(2):525-538. doi: 10.1007/s00253-020-11028-6. Epub 2021 Jan 4. Appl Microbiol Biotechnol. 2021. PMID: 33394152 Review.
-
In-Depth Investigation of Electrostatic Interaction-Based Hydrogel Shrinking for Volumetric Printing and Tissue Engineering Applications.Biomacromolecules. 2025 Jul 14;26(7):4108-4123. doi: 10.1021/acs.biomac.5c00117. Epub 2025 Jun 15. Biomacromolecules. 2025. PMID: 40518730 Free PMC article.
References
-
- Sun H.; Kabb C. P.; Sims M. B.; Sumerlin B. S. Architecture-transformable polymers: Reshaping the future of stimuli-responsive polymers. Prog. Polym. Sci. 2019, 89, 61–75. 10.1016/j.progpolymsci.2018.09.006. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

