High sensitivity LC-MS profiling of antibody-drug conjugates with difluoroacetic acid ion pairing
- PMID: 31500514
- PMCID: PMC6816370
- DOI: 10.1080/19420862.2019.1658492
High sensitivity LC-MS profiling of antibody-drug conjugates with difluoroacetic acid ion pairing
Abstract
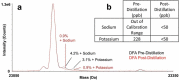
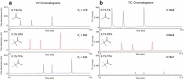
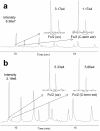
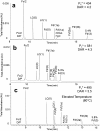
Reversed-phase liquid chromatography (RPLC) separations of proteins using optical detection generally use trifluoroacetic acid (TFA) because it is a strong, hydrophobic acid and a very effective ion-pairing agent for minimizing chromatographic secondary interactions. Conversely and in order to avoid ion suppression, analyses entailing mass spectrometry (MS) detection is often performed with a weaker ion-pairing modifier, like formic acid (FA), but resolution quality may be reduced. To gain both the chromatographic advantages of TFA and the enhanced MS sensitivity of FA, we explored the use of an alternative acid, difluoroacetic acid (DFA). This acid modifier is less acidic and less hydrophobic than TFA and is believed to advantageously affect the surface tension of electrospray droplets. Thus, it is possible to increase MS sensitivity threefold by replacing TFA with DFA. Moreover, we have observed DFA ion pairing to concomitantly produce higher chromatographic resolution than FA and even TFA. For this reason, we prepared and used MS-quality DFA in place of FA and TFA in separations involving IdeS digested, reduced NIST mAb and a proprietary antibody-drug conjugate (ADC), aiming to increase sensitivity, resolution and protein recovery. The resulting method using DFA was qualified and applied to two other ADCs and gave heightened sensitivity, resolution and protein recovery versus analyses using TFA. This new method, based on a purified, trace metal free DFA, can potentially become a state-of-the-art liquid chromatography-MS technique for the deep characterization of ADCs.
Keywords: ADC; DAR; DFA; Difluoroacetic acid; FA; IdeS digestion; LC-MS; MS sensitivity; NIST mAb; TFA; antibody-drug conjugate; disulfide isoforms; drug-to-antibody ratio; formic acid; mAb; metal adducts; monoclonal antibody; peak capacity; potassium; protein recovery; reversed-phase chromatography; salt adducts; sodium; subunit profiling; trifluoroacetic acid.
Figures

Similar articles
-
Residue analytical method for the determination of trifluoroacetic acid and difluoroacetic acid in plant matrices by capillary electrophoresis tandem mass spectrometry (CE-MS/MS).J Chromatogr A. 2021 Jun 7;1646:462096. doi: 10.1016/j.chroma.2021.462096. Epub 2021 Mar 22. J Chromatogr A. 2021. PMID: 33878620
-
Alternative mobile phase additives for the characterization of protein biopharmaceuticals in liquid chromatography - Mass spectrometry.Anal Chim Acta. 2021 Apr 29;1156:338347. doi: 10.1016/j.aca.2021.338347. Epub 2021 Feb 24. Anal Chim Acta. 2021. PMID: 33781463
-
Practical approaches for overcoming challenges in heightened characterization of antibody-drug conjugates with new methodologies and ultrahigh-resolution mass spectrometry.MAbs. 2018 Apr;10(3):335-345. doi: 10.1080/19420862.2018.1433973. Epub 2018 Feb 20. MAbs. 2018. PMID: 29393747 Free PMC article. Review.
-
Glycine additive facilitates site-specific glycosylation profiling of biopharmaceuticals by ion-pairing hydrophilic interaction chromatography mass spectrometry.Anal Bioanal Chem. 2021 Feb;413(5):1267-1277. doi: 10.1007/s00216-020-03089-3. Epub 2020 Nov 26. Anal Bioanal Chem. 2021. PMID: 33244686
-
The effect of the mobile phase additives on sensitivity in the analysis of peptides and proteins by high-performance liquid chromatography-electrospray mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2005 Oct 25;825(2):111-23. doi: 10.1016/j.jchromb.2005.03.041. Epub 2005 Apr 26. J Chromatogr B Analyt Technol Biomed Life Sci. 2005. PMID: 16213445 Review.
Cited by
-
Development of a Rapid Adeno-Associated Virus (AAV) Identity Testing Platform through Comprehensive Intact Mass Analysis of Full-Length AAV Capsid Proteins.J Proteome Res. 2023 Dec 20;23(1):161-74. doi: 10.1021/acs.jproteome.3c00513. Online ahead of print. J Proteome Res. 2023. PMID: 38123456 Free PMC article.
-
Rapid and Simultaneous Characterization of Drug Conjugation in Heavy and Light Chains of a Monoclonal Antibody Revealed by High-Resolution Ion Mobility Separations in SLIM.Anal Chem. 2020 Apr 7;92(7):5004-5012. doi: 10.1021/acs.analchem.9b05209. Epub 2020 Mar 17. Anal Chem. 2020. PMID: 32142606 Free PMC article.
-
Quantification of Methylene Blue and Evaluation of Its Pharmacokinetics in ICR Mice by Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry Using Difluoroacetic Acid.Biomed Chromatogr. 2025 Jun;39(6):e70080. doi: 10.1002/bmc.70080. Biomed Chromatogr. 2025. PMID: 40254552 Free PMC article.
-
Automated Liquid Handling Extraction and Rapid Quantification of Underivatized Amino Acids and Tryptophan Metabolites from Human Serum and Plasma Using Dual-Column U(H)PLC-MRM-MS and Its Application to Prostate Cancer Study.Metabolites. 2024 Jun 30;14(7):370. doi: 10.3390/metabo14070370. Metabolites. 2024. PMID: 39057693 Free PMC article.
-
Characterization of Adeno-Associated Virus Capsid Proteins with Two Types of VP3-Related Components by Capillary Gel Electrophoresis and Mass Spectrometry.Hum Gene Ther. 2021 Nov;32(21-22):1403-1416. doi: 10.1089/hum.2021.009. Epub 2021 Jul 16. Hum Gene Ther. 2021. PMID: 34082578 Free PMC article.
References
-
- Schiel JE, Mire-Sluis A, Davis DL . Monoclonal Antibody Therapeutics: The Need for Biopharmaceutical Reference Materials. In: Shiel JE, Davis DL, Borisov OV, editors. State-of-the-Art and Emerging Technologies for Therapeutic Monoclonal Antibody Characterization Volume 1. Monoclonal Antibody Therapeutics: Structure, Function, and Regulatory Space. Washington (DC): The American Chemical Society; 2014. p. 1–34.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
