Choroid plexus perfusion and intracranial cerebrospinal fluid changes after angiogenesis
- PMID: 31500523
- PMCID: PMC7370367
- DOI: 10.1177/0271678X19872563
Choroid plexus perfusion and intracranial cerebrospinal fluid changes after angiogenesis
Abstract
Recent studies have provided evidence that cortical brain ischemia may influence choroid plexus function, and such communication may be mediated by either traditional CSF circulation pathways and/or a possible glymphatic pathway. Here we investigated the hypothesis that improvements in arterial health following neoangiogenesis alter (i) intracranial CSF volume and (ii) choroid plexus perfusion in humans. CSF and tissue volume measurements were obtained from T1-weighted MRI, and cortical and choroid plexus perfusion were obtained from perfusion-weighted arterial spin labeling MRI, in patients with non-atherosclerotic intracranial stenosis (e.g. Moyamoya). Measurements were repeated after indirect surgical revascularization, which elicits cortical neoangiogenesis near the revascularization site (n = 23; age = 41.8 ± 13.4 years), or in a cohort of participants at two time points without interval surgeries (n = 10; age = 41.7 ± 10.7 years). Regression analyses were used to evaluate dependence of perfusion and volume on state (time 1 vs. 2). Post-surgery, neither CSF nor tissue volumes changed significantly. In surgical patients, cortical perfusion increased and choroid plexus perfusion decreased after surgery; in participants without surgeries, cortical perfusion reduced and choroid plexus perfusion increased between time points. Findings are discussed in the context of a homeostatic mechanism, whereby arterial health, paravascular flow, and/or ischemia can affect choroid plexus perfusion.
Keywords: Cerebrospinal fluid; arterial spin labeling; choroid plexus; glymphatic; ischemia; perfusion.
Figures
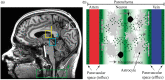

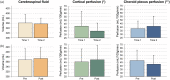
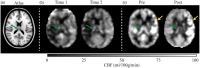

Similar articles
-
Choroid plexus perfusion in sickle cell disease and moyamoya vasculopathy: Implications for glymphatic flow.J Cereb Blood Flow Metab. 2021 Oct;41(10):2699-2711. doi: 10.1177/0271678X211010731. Epub 2021 Apr 28. J Cereb Blood Flow Metab. 2021. PMID: 33906512 Free PMC article.
-
Non-invasive measurement of choroid plexus apparent blood flow with arterial spin labeling.Fluids Barriers CNS. 2020 Sep 22;17(1):58. doi: 10.1186/s12987-020-00218-z. Fluids Barriers CNS. 2020. PMID: 32962708 Free PMC article.
-
Choroid plexus perfusion and bulk cerebrospinal fluid flow across the adult lifespan.J Cereb Blood Flow Metab. 2023 Feb;43(2):269-280. doi: 10.1177/0271678X221129101. Epub 2022 Oct 6. J Cereb Blood Flow Metab. 2023. PMID: 36200473 Free PMC article.
-
Choroid Plexus Aquaporins in CSF Homeostasis and the Glymphatic System: Their Relevance for Alzheimer's Disease.Int J Mol Sci. 2023 Jan 3;24(1):878. doi: 10.3390/ijms24010878. Int J Mol Sci. 2023. PMID: 36614315 Free PMC article. Review.
-
Review of Cerebrospinal Fluid Physiology and Dynamics: A Call for Medical Education Reform.Neurosurgery. 2022 Jul 1;91(1):1-7. doi: 10.1227/neu.0000000000002000. Epub 2022 May 10. Neurosurgery. 2022. PMID: 35522666 Review.
Cited by
-
Choroid plexus aging: structural and vascular insights from the HCP-aging dataset.Fluids Barriers CNS. 2024 Dec 5;21(1):98. doi: 10.1186/s12987-024-00603-y. Fluids Barriers CNS. 2024. PMID: 39639335 Free PMC article.
-
Choroid plexus perfusion in sickle cell disease and moyamoya vasculopathy: Implications for glymphatic flow.J Cereb Blood Flow Metab. 2021 Oct;41(10):2699-2711. doi: 10.1177/0271678X211010731. Epub 2021 Apr 28. J Cereb Blood Flow Metab. 2021. PMID: 33906512 Free PMC article.
-
Arterial spin labeling signal in the CSF: Implications for partial volume correction and blood-CSF barrier characterization.NMR Biomed. 2023 Mar;36(3):e4852. doi: 10.1002/nbm.4852. Epub 2022 Nov 9. NMR Biomed. 2023. PMID: 36269104 Free PMC article.
-
The Regulation of Cerebral Spinal Fluid Flow and Its Relevance to the Glymphatic System.Curr Neurol Neurosci Rep. 2020 Oct 19;20(12):58. doi: 10.1007/s11910-020-01077-9. Curr Neurol Neurosci Rep. 2020. PMID: 33074399 Free PMC article. Review.
-
Regional Glymphatic Abnormality in Behavioral Variant Frontotemporal Dementia.Ann Neurol. 2023 Sep;94(3):442-456. doi: 10.1002/ana.26710. Epub 2023 Jun 14. Ann Neurol. 2023. PMID: 37243334 Free PMC article.
References
-
- Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis 2011; 128: 309–316. - PubMed
-
- Guyton AC, Hall JE. Textbook of medical physiology, 11th ed. Philadelphia: Elsevier Saunders, 2006.
-
- Emerich DF, Vasconcellos AV, Elliott RB, et al.The choroid plexus: function, pathology and therapeutic potential of its transplantation. Expert Opin Biol Ther 2004; 4: 1191–1201. - PubMed
-
- Faraci FM, Mayhan WG, Heistad DD. Effect of serotonin on blood flow to the choroid plexus. Brain Res 1989; 478: 121–126. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

